Table of Contents
Methods used to determine the quantity of gold present in a sample vary according to the type of prospecting being done, and the manner in which the work is carried out. These methods can be classified as:
- dry-blowing
- panning
- other gravity concentration methods such as jigging, tabling and cradling
- centrifugal concentrating
- amalgamating
- spectrographi analysis
- fire assaying
- chemical testing
Dry-blowing
Dry-blowing of powdered samples is practised where water is extremely scarce. Harris (1920) mentions shaking mechanisms designed to work on the same principle, and some variations include bellows or blower to give a more constant air velocity. A process, which depends for its effectiveness on a variable factor such as the wind, tends to be inaccurate, and as well as that it is very dusty work. The method is therefore used mainly by individual prospectors in desert areas and can be expected to detect only coarse gold.
Panning and other gravity methods of concentrating
For assessing values in gravels, soils or outcrops, the miner’s pan, when used by an experienced prospector, is a tool that can hardly be surpassed for simplicity, speed and sensitivity, and a high degree of accuracy can be achieved. For general prospecting the only equipment required is a miner’s dish, a shovel, and at least 20 gal. of water. Many accessory items such as a pick, dolly pot, etc., are often used, but even so the equipment is cheap and simple. Under good conditions, samples of alluvium or loam can be dug and panned off at a rate of six to ten per man hour, though if gold is very fine this figure will be reduced to two or three pannings. In a 10 lb. sample it is just possible to detect a single colour of gold weighing as little as 1/50 mgm. This is equivalent to a limit of sensitivity of one in-225 million or a value of 0 003 dwt. per long ton.
Accuracy of visual estimation of grade varies widely with experience of operators, and usually the pan is regarded as an indicator of the presence of gold rather than a means of estimating it quantitatively. If a high degree of accuracy is required however, it is necessary only to weigh or measure each sample and weigh the final concentrate.
As a result of its sensitivity the pan can be used to follow a very thin trail of golden stream sediments when searching for a greater concentration. Again it can be used in a loaming technique as a soil sampling tool.
If gold is attached to other minerals, as in rock from an outcrop, the sample can be suitably reduced in grain size in a dolly pot. If there is a possibility of tarnish reducing the visibility of gold, any coating can usually be removed, after concentration, with a little commercial HCl.
The pan or other gravity concentration devices, such as cradle, riffles, jig or any type of table, can be used to make field assays of alluvial borehole samples. These devices do not catch very fine gold, but as most large scale alluvial concentrating equipment suffers from the same defect the error is not detected unless check samples are fire assayed. In practice these sampling devices give recoverable gold in a deposit.
The efficiency of any assaying method dependent on gravity concentration is limited by the size of the gold particles. As an example, for gold to be saved-by panning and for it to be seen at the end of the process, it must obviously be of visible size. If the grain size of the gold is any finer than what is usually termed flour, gold—about the size of a pin’s point it will be washed, out with the rock and will not be detected.. In rapid panning too, flour gold will be lost. Similarly in the other methods mentioned above the size of fine gold that can be recovered depends on the degree of turbulence encountered.
Centrifugal concentrating
For bulk testing alluvial deposits, a simple type of mobile centrifugal concentrator has been used by Girand (1936). This type of unit is driven by a small petrol engine and fed by a mechanical shovel. For testing a large deposit this, expensive equipment would be thoroughly justified, particularly if values were thought to be irregular. It does not appear suitable for prospecting anything but alluvial wash.
Amalgamating
Amalgamating of free clean gold in a sample can usually, be achieved by rolling the sample in a barrel with a small charge of mercury. Subsequently the mercury can be separated and distilled, to leave a gold sponge which must be weighed but this method is not always efficient for very fine gold which, may not amalgamate readily. Both flouring of the mercury and tarnished coatings on the gold can cause inaccuracies.
Spectrographs analysis
In a well organized and properly equipped laboratory spectrographical analyses can be done rapidly and cheaply and this method will give accurate estimates of even very small quantities of gold as confirmed by Lundberg (1941) and Konovalov (1941). A disadvantage of this method is that the capital cost of setting up such a laboratory is high, according to Bichan (1958), and would not be justified for preliminary reconnaissance work. For a large prospecting campaign the equipment would be suitable provided a very uniform distribution of values could be achieved in samples.
Fire assaying
Fire assaying gives a high degree of accuracy on any type of sample, except colloidal gold, provided a large enough sample is taken for example, taking 10 assay tons of sample, the result can be reported to ± 0.010 dwt/ton. It is, however, expensive to use for a prospecting campaign that might include many low grade or waste samples. Costs per sample vary from £1 to £3/3/- each according to the number being run and the location of the assaying.
Chemical testing
There are several well known chemical methods of testing for gold-in solutions or in a mineral specimen, but there appear to be only two methods that have been adapted for prospecting these are the dithizone method and the gold mirror test which will both be discussed in the next section under “Methods Investigated”.
The reason for lack of interest in chemical methods for gold assaying is that most orebodies shed particles of free metal of a size that can be readily detected by other means. A chemical method, or fire assaying becomes necessary only when looking for trace quantities or when the gold is all so fine that it cannot be detected by panning.
Fine gold in Victoria is most unusual, but some outcrops of this nature have been located. In an effort to determine the reason for this fine distribution, Edwards and Williams (1958) examined soft leached specimens under high power magnification but were unable to locate any particles of gold in a 22 dwt/ton sample. Only after passing 25 gm. through a superpanner did three specks show up; the largest of these measured 28 x 15 microns. They concluded that the gold in the slate occurs as minute free particles which were probably associated originally with, pyrite. They point out that gold could be unrelated to pyrite but think it unlikely.
However, the manner in which assays of the material can be repeated time and again with exactly the same result each time shows that the distribution is extremely even. It seems possible that gold ions could be loosely attached to clay molecules as demonstrated by Endell (1949).
As such fine gold could easily have been missed even by experienced prospectors, it was decided to investigate other methods of field assay, particularly chemical methods.
Methods Investigated
Gold is not easy to get into solution. There are few satisfactory solvents, and even the well known ones such as aqua regia and cyanide take an appreciable time to- get the metal into solution.
A number of tests have been developed to detect gold under special circumstances. A chemical method has obvious advantages where fine gold is concerned, provided the method and equipment can be kept simple and the time per sample kept low.
When panning at a rate of 10 samples per hour it is usual to detect gold in samples assaying upwards from 0-1 dwt/ ton provided the grains are visible. This is equivalent to a sensitivity of 1 in 6,500,000 which, though very useful, was thought to be more sensitive than necessary when prospecting. It was decided to aim for a lower limit of sensitivity of 1 in 650,000 and an upper limit of about 1 in 33,000; i.e. 1 to 20 dwt/ton.
A solvent extraction process, in which gold would show up in a definite layer of a characteristic colour, was considered desirable. One method used by Polucktov (1943) has a sensitivity ranging from one in 25 million to one in 50 million, but this was too far outside the desired range.
The dithizone method has found wide application in prospecting for copper, lead and zinc, and is now being used for many other metals. The speed with, which assays can be made and the simplicity of equipment is most attractive so that this was an obvious line of investigation to follow. Fischer (1934) established that dithizone in acid solutions will give a pale brown or yellowish reaction with gold solutions and that the gold will displace Hg, Ag, and Cu, and will be displaced itself by Pd. Then in 1939 he stated that “possibilities of quantitative gold estimation have not yet been investigated in detail”. Preliminary tests on the lines mentioned have shown that excess iron can be a problem.
V. P. Sokolof (1950) used the dithizone method in Western Australia and called it “the Kalgoorlie gold test”, but no details were given from which quantitative tests could be worked out. His diagrams showed merely four ratings— high, medium, low and absent.
T. S. Lovering (1955) gives a list of fifteen metals for which the dithizone method has proved successful, but gold is not included amongst these.
As it appeared that much has still to be done to establish correct solutions needed to give the desired range of sensitivity, work on the dithizone method was postponed until other methods had been considered.
There is a qualitative method (Voss 1957, personal communication) whereby gold can be precipitated from acid chloride solutions as a gold soap and then dissolved in dibutyl amine to give a deep red colour. When ferric iron is present this also gives a similar red colour, which tends to make the result unreliable. If copper is present this will show up as a blue colour, so that copper and gold will give a purple reaction. This method could probably be developed to give a quantitative technique, but the time involved would be considerable.
The gold mirror test (Anon, 1947). is a fairly rapid and simple test for determining gold in pyrite, e.g. a pyritic concentrate from pannings. A 10 per cent tincture of iodine is used to dissolve the gold, which is then reprecipitated as a metallic mirror on a heated pyrex watchglass. However, to get a semi-quantitative result the process may have to be repeated several times; tests are normally done separately; the operator must avoid inhaling iodine fumes as he drops the solution on the watchglass; and finally even a small amount of sediment in the solution will cloud the mirror effect.
Blowpipe tests with plaster tablets were tried, but too much interference occurred to recognize any purple to rose colour which indicates the presence of gold.
Microchemical tests as described by Short (1940) were not tried as they appear to be unsuitable for this type of work, a microscope being an essential part of the equipment.
Sodium carbonate fusion was investigated, but to get a recognisable button requires such a large sample, so much heat and so large a carbon block that a portable assay furnace would be a better proposition.
The B.D.H. spot testing method using benzidine is simple but not sufficiently specific.
Finally chromatography was considered, as this appeared to offer better opportunities for simplification than many other methods. This proved to be correct, as even preliminary tests showed a good degree of differentiation and within four months a paper chromatographic method was developed. This method is simple, to use and reliable within its limits.
Description of Paper Chromatographic (P.C.) Method
The development of this method has been described by Lever (1958), but is summarized here.
Oxidized samples
The sample is ground to 65 or 100 mesh Tyler, avoiding, as far as is reasonable, contamination with metallic iron. Highly silicious samples ground in steel pulverizers show up to 3 per cent metallic iron contamination. If this is not removed by a magnet the efficiency of the leaching process will be impaired.
Strips of Whatman No. 3MM chromatography paper are cut from rolls 1½ in. wide to lengths of 7 in. and marked with a pencil at 1- in., 3¼ in., and 4½- in. from one end. (Rolls are preferred to cut sheets since the paper remains cleaner.) An aluminium frame as in Fig. 1 is placed with its lower edge on the 1- in. mark. This frame is filled with sample which, is compressed to remove excess pores and smoothed
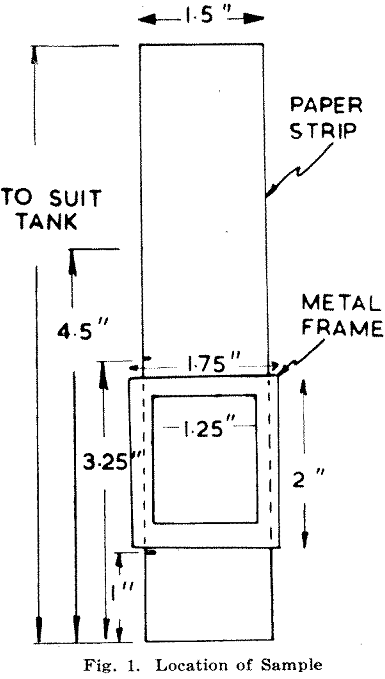
off level with a spatula. This leaves about 14 to 15 gm. of sample on the paper after the frame has been removed.
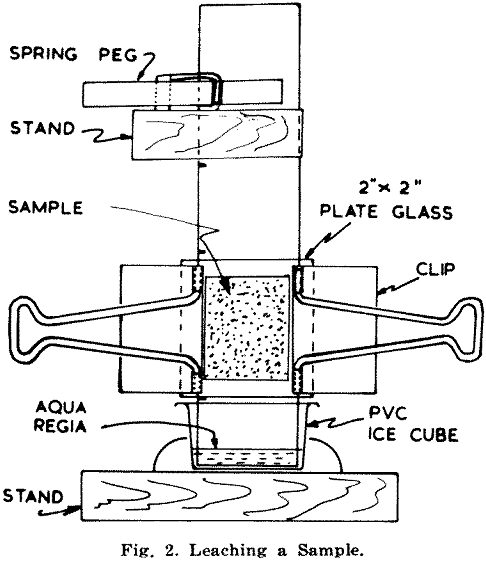
Plate glass squares measuring 2 in. x 2 in. are then clamped on either side with spring clips, and the unit is suspended with its projecting 1 in. dipping into a container holding diluted aqua regia to a depth of about ¼ to 3/8 in. (Fig. 2). Details of solutions used are given in the Appendix.
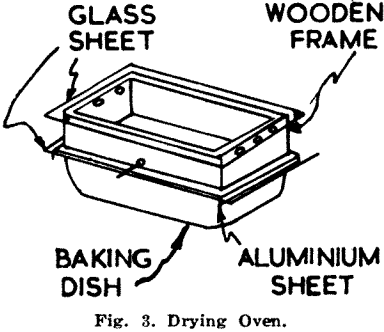
The acid should take about 30 min. to rise through the sample to the 3¼ in. mark, when the unit is removed from the bath. The clips and glass are then removed from the paper, usually leaving most of the sample attached to the glass. The paper strip is placed in an oven (Fig. 3) to dry at a temperature between 55°C and 65°C. After fifteen minutes the strips are removed from the oven, brushed as clean as possible and returned to it for a further ten minutes. After drying the strips are brushed completely clean.
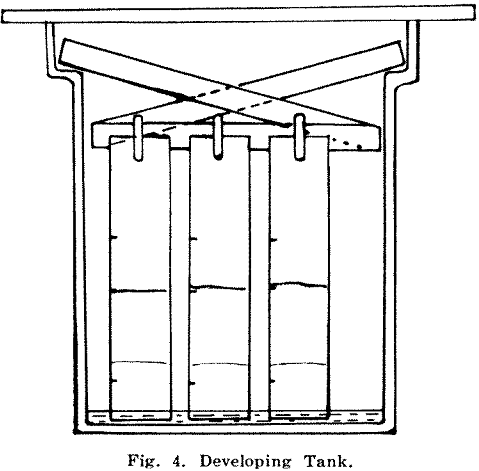
The next stage is to attach the paper strips to a holder and place a batch of about six at a time into a developing tank (Fig. 4) containing 100 ml. of ethyl acetate-nitric acid developing solution. This solution collects any gold as it rises and the front (the advancing edge of the solution) reaches the 4½ in. mark in about 20 min.
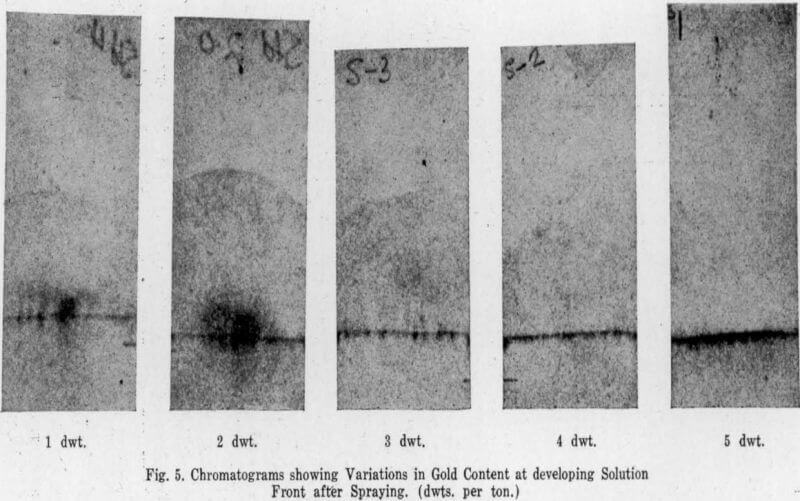
The strips are then removed, placed on a wooden stand, air dried for a few minutes in a warm place and sprayed with fresh stannous chloride solution. Any gold will show
up as a brown or purple line at the developing solution front. Warmth at this stage helps to show up the result within two to five minutes, but too much heat will bring up strong iron colours.
The thickness of the line and the depth of the colour vary with amount of gold in the sample as shown in Fig. 5, so that an estimate can be made within the range of 1 to 10 dwt/ton in steps of 1 dwt. Should a sample be richer than 10 dwt. the line will appear to drag in places and the solution will not carry all the gold to the front. By taking a half frame or a quarter frame of sample, an estimate can be made of ores richer than 10 dwt., since the quantity of gold extracted onto the paper is proportional to the amount of sample used. By doubling the weight of sample used, improved resolution of gold in low grade material should be obtained, but no work has been done on this variation of the method.
Samples carrying sulphides
Sulphides in a sample interfere with the extraction by aqua regia and must therefore be removed.
To do this about 10 gm. of finely ground sample is lightly consolidated in a measuring cylinder to ascertain the volume. The sample is placed in an open dish and roasted for about
an hour at:550°C with constant rabbling. After cooling the calcine is tipped into a 400 ml. beaker and concentrated hydrochloric acid is added in order to remove excess iron. This is then boiled gently for half an hour. The ferric chloride solution is then diluted with water, the residue settled and the solution decanted. Three washes with water are then given in a similar manner, after which the residue is dried on a hot plate. When cool the residue is returned to the measuring cylinder and made up to original volume with clean barren material ground to -100 mesh. Though this correction for volume is not theoretically exact, it is simple. In practice it has given satisfactory results and is considered sufficiently accurate for any work on which the paper chromatographic method would be used. Some examples are shown in Table 1.
After this correction for volume has been made, procedure is exactly the, same as for oxidized samples.
Very heavy concentrations of iron oxide in a sample such, as a limonite outcrop may also prevent efficient movement of gold on the paper. In these cases a leach with hydrochloric acid and replacement of any volume lost will give similar results to those with sulphides.
The method was developed on a battery tailing which contained 5-5 dwt. of gold per ton, and it worked well on this material in the laboratory. To test the method under field conditions, a small prospecting campaign was started in an area where is was. known that some ultra fine gold occurred in decomposed slates. Twenty years earlier four, samples from the area assaying 5 to 67 dwt. per ton had been tested. At the same time it was stated that no gold, was visible on panning. No records of the old claims were available at the Mines Department and no local inhabitant could suggest any more than a vague location, so that this appeared a suitable testing ground for the new method.
Field Trials
To test the assaying method in the field, a complete set of equipment as listed in the Appendix was made up; this cost about £6 in all excepting the gas supply. Bottled gas is not essential, but its convenience makes it well worth while. This equipment was set up at an abandoned school near the site.
In the first trials a quick traverse of the area was made in an attempt to pick the gold bearing outcrop visually. Several slates, similar to the description in the early records, were found and sampled but all proved to be under 0-6 dwt. In the second attempt, some of the hundreds of shaft dumps in the area were sampled by taking hand specimens of any different types of slate visible and testing them. Only one gave a high reaction (about 12 dwt.), but this was sufficient to indicate the correct location. The next batch of outcrop samples located auriferous slate three to four feet wide at three sections along a strike length, of 200 feet.
Trenches have since been excavated in the hanging and footwall sides of this band without finding any repetitions of values, though similar slates were located. Up to February 1959 no more work had been done across the higher grade zone so that extensions of this still remain to be proved.
Comments on the field testing are divided chronologically into the various steps.
Sample Preparation:
The amount of gold taken into solution with aqua regia depends on temperature and time of contact. Temperature control is discussed under “Acid leach”. Contact time should be about 30 min., in order to get an assessable mark after spraying. It is preferable to keep this time constant for different ores. Gritty samples will be more porous than a talcy slate and the acid will travel faster through the porous one. By grinding a gritty sample to 100 mesh Tyler and a slatey sample to 65 mesh, approximately the same porosity and therefore the same leaching time can be achieved.
Compared with the assay ton of 32-66 gm. a 1-4 gm. sample is a small amount on which to base an estimate of the grade of perhaps one to five feet width of ore, but with finely disseminated gold it has proved to be more than adequate. High grade samples were used later as standards. Only a quarter frame or 0-35 gm. was used many times from the same sample, and each time it gave almost exactly the same result.
On samples where gold occurs more coarsely, variations do occur but so far have’ not been serious, the maximum being about 2 to 3 dwt. in a 5 to 6 dwt/ton sample.
Acid Leach (Fig. 2):
Two types of stands were used; the more satisfactory one allowed individual adjustment of each paper. Glass beakers for holding acid were superseded by PVC ice cube containers 1½ in. long by 1 in. wide. Only about a quarter inch depth of acid is required for each run, and if clean it can be used again. Should it become contaminated by a little sample falling off the paper, the loss of acid is very small.
As mentioned before, air temperature at this stage is important and should not be allowed to fall below 18 °C or 64° F. At low temperatures insufficient gold is picked up in the 30 min.; also the activity of the developer is reduced, impairing the general efficiency of the process. This is indicated by the lack of a definite band at the solvent front after spraying. In cold weather therefore it is necessary to warm the room where determinations are made. The method works perfectly in air temperatures up to 90°F but no observations have been made above this.
Drying (Fig. 3):
The glass sheets in the oven and the spare sheet outside used for brushing off the samples must both be washed clean after each batch of samples to avoid contamination in the wet stage. Ample ventilation in the first 15 min. of drying is essential.
Temperatures of drying are critical as already explained by Lever (1958) and best results were obtained between 55°C and 65°C. The simple type of oven gives remarkably even temperatures, particularly if it is heated up to 70° and then allowed to cool with practically no flame under it. Another way to improve the stability of the oven is to stand an empty tin of about 1 gallon size over the flame and place the oven over the tin. This spreads the heat so evenly that variations of only 1°C over the 25 minutes can be attained.
If much sample is left on the paper before inserting in the oven, drying will be retarded. It is desirable therefore to remove most of this material after 15 min., making sure the surface of the paper is not damaged. When wet the paper is fragile, but when dry can be handled quite freely.
Developing:
Initially aluminium rods were used to support stainless steel spring clips which held the paper strips in the developing tank. Aluminium did not prove satisfactory as corrosion took place and small crystals of aluminium salts dropped into the developing solution. Wooden paper holders with plastic show card clips have since proved quite satisfactory.
The ratio of ethyl acetate, water and nitric acid in the developing solution is critical, and the introduction of any foreign salts which may react with this mixture, interferes with this balance and renders, the developing process inefficient.
Spraying:
Stannous chloride solution is corrosive, so that the operation should be carried out separately if possible. A light spray on all papers is given first to fix the gold. This is followed by a heavier spray on both sides of the paper, to bleach out iron. If there is. any gold in the sample it should show up within two to five minutes while warming gently. Low grade samples are slower to show than high grade. Over-heating at this stage will bring up strong purple and brown colours which can be mistaken for gold, but a second spraying will wash this colour away. Any marks due to gold will be permanent.
Standards for comparison are easily made by running a known sample with the first batch each day. This has the advantage of testing all the solutions at the same time. Permanent standards, as shown in Fig. 5, are useful to have on record, but the daily standard is a valuable check on solutions and should always be used. When dealing with low grade samples it is often worth while to run a blank as well.
Faulty operation or faulty solutions can usually be detected when spraying. For instance, if gold has precipitated due to too high a temperature in the oven there will be no resolution of the gold, as the ethyl acetate developing solution will not dissolve metallic gold and carry it forward up the paper. Under-drying, which means the introduction of hydrochloric acid, disturbs the acid ratio of the developing solution. The migration of the gold complex will still occur but it will not travel at the solvent front. The gold will then show after spraying as a diffused zone at some distance below the solvent front. With a reasonably high gold bearing sample, if the paper strip contains an excess of hydrochloric acid, the gold can be seen to have diffused through the whole length of paper through which the developing solution has passed.
Accuracy of estimation:
For a field method the accuracy is high over the greater part of its range the method has an accuracy on oxidized samples of better than 75 per cent, and, as shown in Fig. 6, the change-over from full frame samples to half or quarter frame causes no serious deviation of the curve.
Samples carrying sulphides are not included in the above statement because, though the accuracy so far obtained in
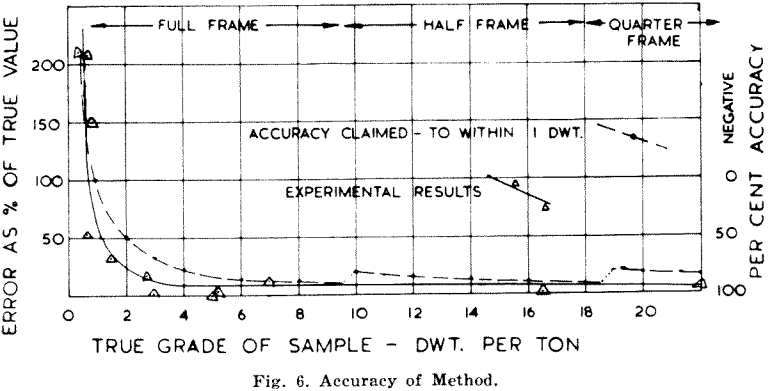
practice is in the same range, the adjustment of volume rather than weight ought to cause a greater deviation than it does. This anomaly is thought to be due to compensations brought in while cleaning the sample with acid, but so far no time has been available to prove this point.
Comparisons against fire assays are shown in Table 1—the first section for high assays, the second for low assays, and the third section for sulphides. The comparisons against high assays are reasonably close. The second section relates to a group of tailing dump samples and the comparison indicates a tendency to over-estimate at the bottom of the range. The error in this case is nearly 200 per cent, which
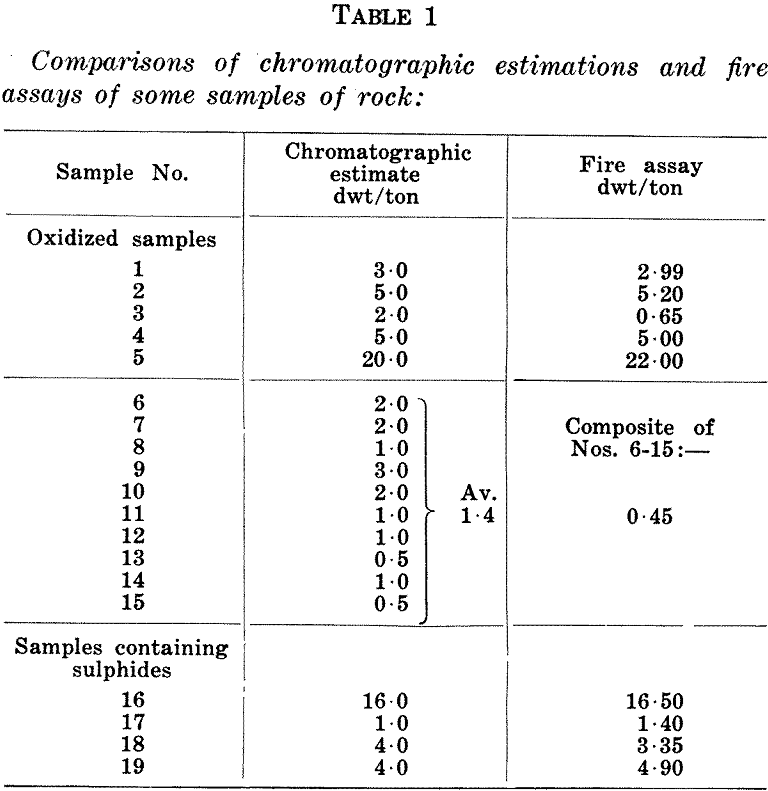
appears high but actually the averages coincide within the limits of the method, namely to ±1 dwt.
Portability:
All gear required for prospecting, including pick and shovel and gas equipment if desired, can be packed into the boot of an average car. A Land-Rover would take everything with room for camp equipment as well. The glassware has been transported 100-miles over fairly good roads without breakages. It is, however, desirable to have spare units such as a spray, glass sheets, and plate glass-top for developing jar, if travelling any distance from supplies.
Operating conditions:
Sample preparation requires a bench space of about 6 ft. x 2 ft; preferably separated from the assaying equipment and well ventilated.
Assaying equipment can be handled on another bench 6 ft. x 2 ft., with additional space for a sink and small draining board. The space for the oven depends on the heat supply used but if it is a small unit, such as a bunsen burner, a space of 2 ft. x 1 ft. 6 in. is sufficient.
Stability of solutions:
The three solutions used have been tested intermittently over a period covering the greater part of two years. No special precautions are necessary to keep them in good condition.
Aqua regia 1:1 keeps indefinitely in glass stoppered bottles. The developing, solution is formally left in the tank while in daily use. If the solution is not to be used for some time it is necessary to pour it back into a glass stoppered bottle to avoid evaporation. It can be stored in this way for months without losing effectiveness.
Stannous chloride will keep in fresh condition in a glass stoppered bottle for some months provided a little metallic tin is left in the bottle. This solution is never left overnight in the spray bottle as it will start to oxidize, nor is tin allowed to get into the spray bottle since fragments of metal might block the fine jet.
Costs:
By using paper stands made from scrap timber all the equipment plus a supply of reagents can be purchased for under £10.
Running costs for this method are largely labour charges so that the daily number of assays and wages of operator determine the cost. With equipment to take six papers at a time, one man can do 35 to 40 determinations and a standard in hours actual working time.
Sample preparation depends on rock hardness, but with well decomposed slate 30 to 40 samples can be quartered, crushed, riffled and ground to 100 mesh in a dolly pot, in a hour working time. With suitable mechanical aids this figure could be improved.
When dealing with rock which has its gold content exceedingly finely divided, sampling has few problems. In the case under review samples of 5 to 10 lb. were taken and treated in a normal manner, but from the regularity with which the standard result can be repeated, there is every indication that small samples would be just as effective and much quicker to handle.
On the basis of labour at £4 a day, 40 samples prepared per man shift and 40 samples assayed per man shift, cost would be

Cost of treating sulphide samples is 50 per cent above this total.
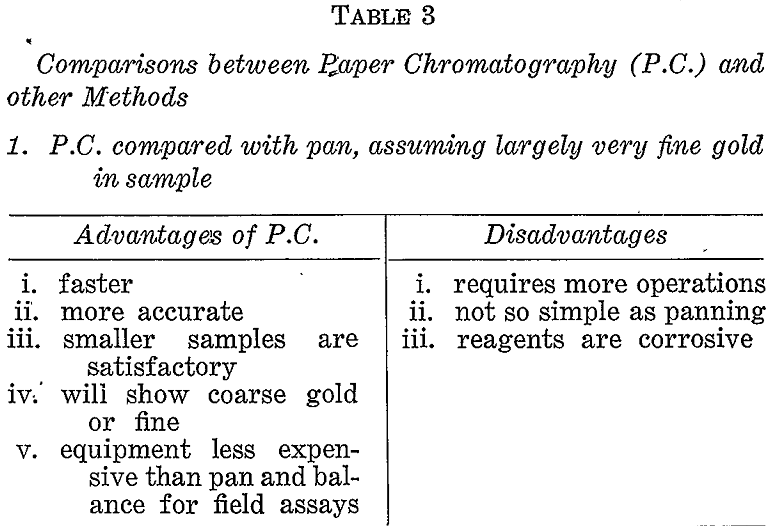
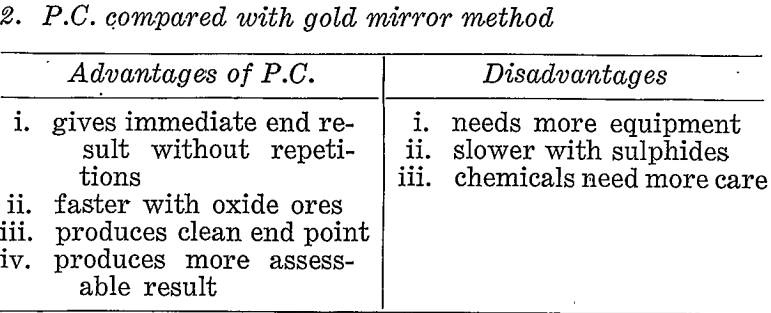
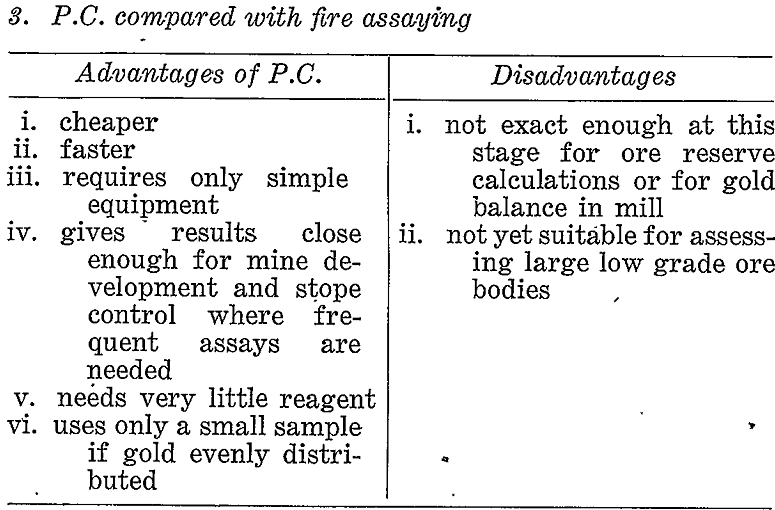
Comparisons With Other Methods
If prospecting for coarse gold the pan or some suitable variation of gravity concentration would be the correct choice. If, however, a large proportion of the gold might possibly be fine then other methods must be considered. Table 3 briefly compares the paper chromatographic, method with panning, gold mirror method, and fire assay under these conditions.
Conclusions
The chromatographic method provides a relatively rapid inexpensive determination for gold in any rock where 1½- gm. of sample can be considered representative.
For oxidized samples the method is sufficiently simple for anybody to operate after a little instruction.
For sulphide samples a little more training is needed, but no operation calls for any chemical background.
Simple field equipment for roasting sulphide samples has not yet been developed.
The equipment needed is inexpensive simple to handle, not unduly fragile, and can be transported by any car.
The solutions used are stable but must be made up carefully.
The method is quite satisfactory for prospecting within the limits of 1 to 40 dwt/ton but for estimations of lower grade material greater sensitivity is required.
As well as this it appears quite suitable for development and grade control work in a mine, and may have some applications in mineral dressing. Geologists should also find it useful for testing small specimens.
