Evidence for the floatability of sulphide minerals without collector is analyzed and it is shown that results obtained with ores, in contrast to those with single minerals or mineral mixtures, depend on many additional variables, some identified and others not. Because of the prevailing low levels and costs of collectors for most sulphide ores, it is unlikely that collectorless flotation will have commercial applications, so that continued research on the subject may be justifiable on theoretical but not on practical grounds.
The key factor is the identification of near neutral sulphur atoms in the mineral surface with the hydrophobic/floatable condition. For chalcopyrite, the” hydrophobic entity produced by slight oxidation is probably sulphur-based but whether it is sulphur itself, sulphur enriched sulphide, (or) a metal poly sulphide, is of no particular consequence and indeed may be different in different circumstances or for different minerals” (Shannon & Trahar 1986).
The system properties which determine collectorless flotation include:
- Mineral properties
- Mine environment
- Grind environment
- Flotation Environment
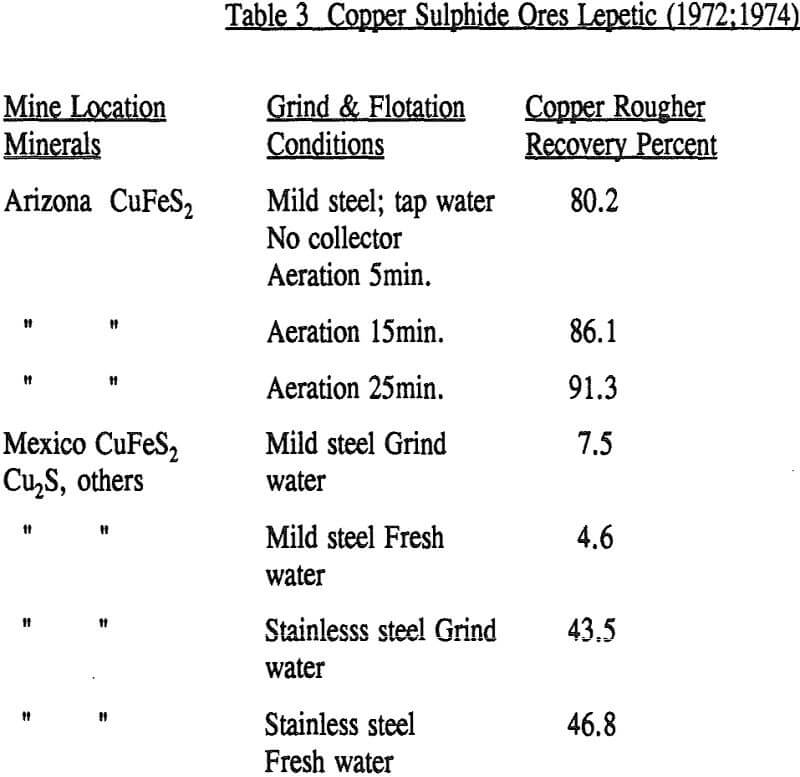
The obvious conclusion from the tests with ores is that, although there is substantial and incontrovertible evidence for collectorless flotation of sulphides, this has almost always required a closely controlled environment. While one of the variables requiring this control is the system redox potential as affected by oxidants or reductants, other reactants derived from the ore or from water sources may also need to be controlled, or their effects negated. It has been shown specifically that the same copper mineral may vary widely in response within a particular mine according to rock type; and from one mine to another, for reasons usually not established.