Table of Contents
In support of its goal of maintaining an adequate supply of minerals to meet national economic and strategic needs, the Bureau of Mines has investigated the feasibility of in situ and heap leaching to recover manganese from low- and medium-grade domestic manganese deposits and to also recover the silver found in some of these manganese deposits.
In situ leaching technology has been successfully used for uranium and copper. Heap leaching is an important method for recovery of gold, silver, and copper. These leaching technologies have the advantages over conventional mining of lower capital and operating costs, shorter startup times, and environmental and safety improvements. Thus, in situ or heap leaching is a favorable method for small and/or low-grade deposits that would not otherwise be mined. Most of the more than 2,100 low-grade manganese deposits in 35 States fall into this category.
Previous Bureau research indicated the technical feasibility of recovering manganese from low-grade deposits by in situ leaching with aqueous SO2. Manganese recovery ranged from 60 to 95 pct for oxide and carbonate deposits. As an offshoot of this project, the feasibility of recovering silver and manganese from refractory silver deposits was evaluated. These deposits are refractory because the silver Is either bound with the higher order manganese oxides, rendered inaccessible to cyanide leach solutions by the surrounding manganese mineralization, or chemically resistant to cyanide leaching. The evaluation was accomplished by conducting column-leach tests, using a dual leaching scheme in which silver is leached with cyanide solutions after an aqueous SO2 leach of the manganese and an alkaline rinse to neutralize any residual acid (to assure that no toxic HCN gas forms). The results of this evaluation are described in this report.
Gaspirrini, in a study of the mineralogy of silver and its significance in metal extraction, including that by NaCN solutions, found that factors affecting the metallurgy of silver are (1) the silver-bearing minerals, (2) their grain size, (3) their host minerals, and (4) the association of the host minerals with the silver-bearing minerals. The common silver-bearing minerals native silver, electrum (AuAg), argentite-acanthite (Ag2S), copper-silver minerals, and silver halides such as ceragyrite (AgCl), embolite [(Ag(Br,Cl)], bromyrite (AgBr), and iodyrite (Agl), etc., are readily dissolved in NaCN solutions, particularly when finely divided (<15 µm in size). The common silver-bearing minerals proustite (Ag3AsS3), pyragyrite (Ag3SbS3), stephanite (Ag5SbS4), polybasite [(Ag, Cu)16Sb2S11], tennantite [(Cu, Ag, Fe)12AS4S13], and tetrahedrite [(Cu, Ag, Fe)12Sb4S13] do not dissolve readily in NaCN. Preroasting may improve silver recovery in these cases. Lead-zinc minerals containing silver in solid solution and manganese-silver minerals and solid solutions are leached by NaCN solutions with great difficulty.
Although several laboratory tests have been successfully conducted on leaching of manganese with SO2 solutions followed by leaching of silver with cyanide solutions, the present study has several key differences from earlier studies. In the present study, percolation SO2 and CN leaching tests were conducted on larger ore pieces (minus 2.5- plus 1.3-cm) rather than fine ore particles (<0.210 mm) to simulate in situ and/or heap leaching conditions. A 5- wt-pct-SO2 solution was used to preferentially leach most of the manganese while leaving most of the iron in the residue. In other studies employing high concentrations of SO2 (8 to 9 wt pct SO2), most of the iron was leached along with the manganese. Another difference from past studies is the elimination of calcining of the ore to improve permeability or to decompose carbonates.
Characteristics of Deposits
The manganese deposits that were sampled and tested in the column leaching experiments are characterized in table 1. The deposits are of hydrothermal vein origin and contain predominately the higher order oxide minerals: pyrolusite and psilomelane. The samples were obtained mostly from waste rockpiles and lean ore dumps around mine sites in the listed districts. Chemical analyses of the samples, partially given in table 2, indicated that they contained between 1.8 and 7 tr oz/st Ag.
Experimental Method
Manganese extraction was determined on 3.5-kg samples of minus 2.5- plus 1.3-cm pieces in 10-cm-diam Plexiglas acrylic columns that were 1 m long- A 5-wt-pct- SO2 solution was applied at a rate of 1 mL/min. This rate approximates the application rates in commercial gold-silver and copper heap leaching operations and resulted in an unsaturated column of ore. Effluent from the column was collected and analyzed for manganese, iron, calcium, and silver by atomic absorption (AA) spectroscopy. Although the AA readings are accurate to ±1 ppm, calculated recovery values are probably accurate to 5 pct of the reported values. The tests were usually run until the manganese in the effluent from the column dropped to 1 g/L. Initial manganese concentrations in the effluent ranged from 2.7 g/L for sample AZ-3 to 12.0 g/L for sample AZ-2. At least 50 L of solution was applied to each ore sample during any one test.
After leaching an amount of manganese that would simulate the recovery in a heap or in situ leaching operation (65 to 95 pct), the material remaining in the column was rinsed with a 0.3-wt-pct-Na0H

solution to neutralize any acidity. This was necessary to prevent formation of toxic HCN gas, which occurs if cyanide encounters acidic solutions.
Following the rinse phase, 1 kg of the material was put into another 10-cm-diam Plexiglas acrylic column and leached with about 4 L of solution containing 0.3 wt pct NaCN and 0.3 wt pct NaOH applied at a rate of 1 mL/min. Effluent solution was collected and analyzed for iron, silver, and cyanide by AA methods. Control samples (no SO2 preleach to remove manganese) were leached with cyanide in an identical manner.
Results
Manganese Extraction Tests
In previous column leaching tests, manganese extractions of 70 to 95 pct have been obtained from samples containing pyrolusite, psilomelane, and/or wad.
The manganese-extraction curves as a function of volume of solution applied to the column are shown in figure 1 for the seven silver-bearing samples. The manganese in all the samples except NV-1 was predominately psilomelane and pyrolusite; therefore, it readily leached to the 80- to 95-pct extraction level. The manganese in sample NV-1 was predominately manganite and was leached at a slower rate.
Silver Extraction Tests
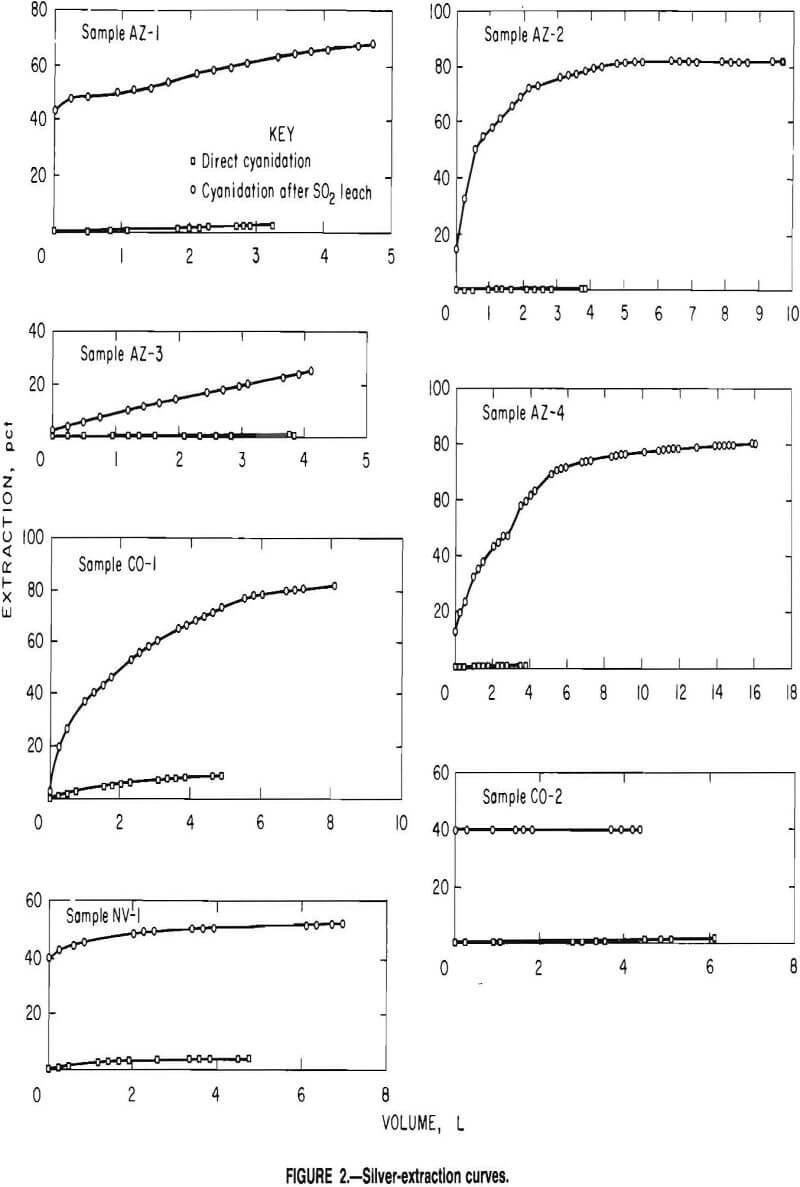
Results of cyanide leaching tests on the control samples and the samples that had been leached with SO2 are summarized in table 3. Silver-extraction rates are displayed in figure 2 for both control and post-SO2 leach samples. Cyanide leaching without SO2 preleaching resulted in silver extractions of 0.7 to 8.5 pct. The SO2 leaching step, intended to remove manganese, also resulted in significant silver extraction in most samples. These

silver values can be recovered either by chloride precipitation or by cementation. Cyanide leaching of the samples after the SO2 leach resulted in additional silver extractions ranging from 2 to 80 pct. The total silver recovered during SO2 leaching and cyanide leaching after the SO2 leach was 8 to 110 times greater than the silver extraction for cyanide leaching alone.
The refractory nature of some silver deposits with regards to extraction by cyanide solutions could be due to several factors. A major factor is the silver mineralization. Some silver minerals are resistant to leaching or leached only to a small extent with cyanide solutions. Another major factor is the intimate association of silver with the manganese

oxides in the ore. It is these deposits that are the target of the methods presented herein; in such deposits the manganese must be dissolved before the silver can be solubilized. Yet another factor is the accessibility of the silver mineralization to the cyanide leach solution.
The linearity of plots of ln [(1+f)/ (1-f)] versus time for SO2 leaching of the ores is exemplified in figure 3 for ores CO-1 and NV-1 and evidenced for all seven ores by the near-unity correlation coefficients for a linear fit of the data (table 4) and proves that the rate data follow the rate expression for autocatalytic reactions:
kt = ln[(1+f)/(1-f)]…………………………………………………………(1)
where k = rate constant,
t = time,
and f = fraction of manganese leached at time t

Discussion
Since the solubilization of manganese upon contact with SO2 was observed to be rapid in previous work, on sea nodules and in batch-leaching tests on fine particles of manganese ores, the rate of leaching of manganese from ores is dependent upon the rate at which the leach solution can penetrate the ore.
Microscopic examination of the ores in this investigation showed that the manganese mineralization had precipitated in and closed capillaries, coated interiors of pores, and precipitated at matrix-grain boundaries. This accounts for the

low water permeabilities observed for these ores (as low as 10-² to 10- 4 darcy) and indicates why most silver mineralization in the bulk of the ore pieces, even though amenable to direct cyanidation, was not leached until after a preliminary SO2 leach. Without removal of manganese to open up pores and capillaries, there is no penetration of any leach solution into the ore pieces.
Since SO2 solutions rapidly leach manganese upon contact, the pores and capillaries are opened up commensurate with the fraction of manganese leached. This is in agreement with the autocatalytic rate expression given in equation 1. Microscopic examination of X-ray diffraction of ore pieces after leaching with SO2 solutions showed that most of the manganese was removed by SO2 leaching.
A parallel situation occurs for ores with high calcite contents. The calcite, like the manganese mineralization, either surrounds the silver mineralization or fills the voids, grain boundaries, and capillaries of the ore, thus preventing cyanide solution from reaching and dis¬solving the silver.
Minerals that fill fissures and pores to such an extent that the solution is physically blocked from attacking the silver can have a profound influence on silver leachability. If manganese is the primary mineral blocking cyanide-solution access to the silver, the SO2 preleach again offers potential.
Clues to the reason for the refractoriness of the manganese-silver ores AZ-1, AZ-2, AZ-3, AZ-4, CO-1, CO-2, and NV-1 are found in the data in table 3 and figure 2, respectively. Silver not solubillzed by the dual-leaching system is probably very slowly leached or resistant to cyanide leaching or is still not accessible to cyanide solutions owing to blockage by calcite or remaining manganese mineralization. Silver cosolubilized with manganese during SO2 leaching is most likely intimately associated with the manganese oxides in the ore. Silver extracted by the cyanide solution after manganese removal by SO2 solutions was previously not accessible to the cyanide solution owing to the presence of manganese mineralization in pores and fluid channels.
About 13 to 14 pct of the silver in ores AZ-2 and AZ-4 (fig. 2) was co-leached with the manganese by SO2 solutions and therefore is intimately associated with the manganese mineralization, while about 67 to 68 pct of the silver was not accessible by cyanide solutions until after the manganese mineralization was removed. The ramaining 18 to 20 pct of the silver is either (1) still encapsulated or intimately associated with the manganese mineralization remaining in the ore, (2) very slowly leached by cyanide, or (3) non-leachable by cyanide. Assuming a homogeneous distribution of the silver throughout the ores, from 1 to 5 pct additional silver should be recovered if the manganese leaching with SO2 is allowed to be completed.
Ore CO-1 (fig. 2) has little silver in the manganese mineralization, as evidenced by the 2 pct of the silver coleached with the manganese (table 3). About 80 pct of the silver was amenable to cyanide leaching after manganese removal, making the silver mineralization accessible to cyanide solutions. Less than 18 pct of the silver is either slowly leached or not leached by cyanide solutions.
Ore AZ-3 (fig. 2) also had little silver in the manganese mineralization (2 pct) (table 3). The linearity of the silver-extraction curves (percent extraction versus volume cyanide solution applied) after 82-pct manganese extraction indicates that much of the refractory nature of this ore is due to the presence of calcite.
Much of the silver in ores AZ-1, CO-2, and NV-1 (fig. 2 is intimately associated with the manganese mineralization as 43, 40, and 38 pct of the silver (table 3) was coextracted with 78, 82, and 75 pct of the manganese, respectively. An additional 25, 14, and 2 pct of the silver was made accessible to cyanidation by removing manganese. Some of the remaining 32, 46, and 60 pct Ag in these ores is probably either slowly leached with cyanide solutions or not leachable with either SO2 or cyanide solutions; however, extraction of more silver is probable if the SO2 leaching of manganese is continued longer. Assuming a homogeneous distribution of silver throughout the ores about 12, 18, and 13 pct additional silver, respectively, would be extracted with the SO2 solutions upon extraction of the remaining manganese.
Conclusions
Column-leaching tests were conducted on minus 2.5- plus 1.3-cm ore pieces to evaluate a dual in situ or heap leaching method for the recovery of manganese and silver from domestic manganese deposits. The method features an initial leach of the manganese with a 5-wt-pct-SO2 solution followed by a neutralization rinse, and then a second leach of the silver with a 0.3-wt-pct-caustic-cyanide solution. Silver-leaching tests were conducted on materials from domestic manganese deposits that contained silver ranging from 1.8 to 7.0 tr oz/st. Direct cyanide leaching of these samples resulted in only 0.7- to 8.5-pct silver extraction. Leaching with cyanide after SO2 leaching resulted in silver extractions ranging from 2 to 80 pct in addition to the 2 to 40 pct recovered during the SO2 leach. Overall silver recovery was 25 to 80 pct.
The dual leaching method appears suit able for deposits in which the silver is intimately associated with the manganese and for deposits in which manganese mineralization prohibits the cyanide solution from attacking the silver.
