Table of Contents
Metal Removal Processes of Wetlands
A wetland environment can remove metals from influent waters in a variety of ways. As chemical oxidation takes place, oxides and hydroxides of iron can be expected to precipitate from surface flow, resulting in the characteristic yellow sediment in mine drainage known as “yellow boy”. These precipitates coat the surfaces of the bottom or settle as a flocculate that subsequently is filtered out as the water passes through the soils and around vegetation. Because precipitation of metals is strongly pH- dependent, and because the pH of mine drainage often is low (usually from 2.5 to 5.5), ferric hydroxide will be the main precipitate, with most other metals remaining soluble in such acid conditions. Where the pH of the water is buffered at higher levels, such as might occur in the presence of carbonate ore bodies, precipitation of other metals will take place, often as co-precipitates with the iron oxyhydroxides. Under conditions of neutral or alkaline pH, precipitation is the dominant form of metal removal, which is one reason that conventional treatment techniques often specify the addition of lime. Under low-pH regimes in wetlands, other processes become more important in removing metal ions.
Bacteria and other microorganisms are important biological components of wetland water and soils. Bacteria, algae, and fungi can affect dissolved metal concentrations by direct accumulation of metals within their body structure or by producing sufficient changes in the aqueous environment to facilitate precipitation of metals from solution. Bacteria in particular have been shown to have the ability to scavenge large amounts of metals from their environment (Beveridge, 1986; Beveridge and Murray, 1976; Dugan, 1970; and Dugan et al., 1970). Sulfate-reducing bacteria are capable of producing metal and hydrogen sulfides, which can be accumulated in the oxygen-poor reducing environment of the sediments. A beneficial side effect of this microbial process is that excess hydrogen ions can be removed by the release of hydrogen sulfide gas, resulting in less acidic conditions (Tuttle et al., 1969).
Practical Limitations to the Use of Wetlands for Mine Drainage Control
While wetland systems offer potential as a treatment medium, there are no long-term scientific studies of their ability to maintain a given level of metal removal, nor has much information been published regarding their cost effectiveness. Investigators from the U.S. Bureau of Mines (Girts and Kleinmann, 1986; Erickson, Girts, and Holbrook, 1987) have summarized the results of studies on a large number of systems installed in the eastern coal mining regions. While there are some obvious successes, most of the systems are only a few years old. Few systems have been constructed in the Rocky Mountain region, and data on their metal removal ability are incomplete. Available land area will more than likely be one of the most important considerations, assuming that ongoing studies substantiate the efficacy of wetland treatment. The Bureau of Mines has been recommending 200 ft² (21.5 m²) of wetland surface area for each gallon per minute (3.79 l/min) of discharge, which would amount to nearly 0.5 acre (2023.4 m²) for a discharge of 100 gpm (378.5 l/min). Even that amount of area may not be enough if the site is at a relatively high elevation where cooler climate would slow geochemical and biological processes.
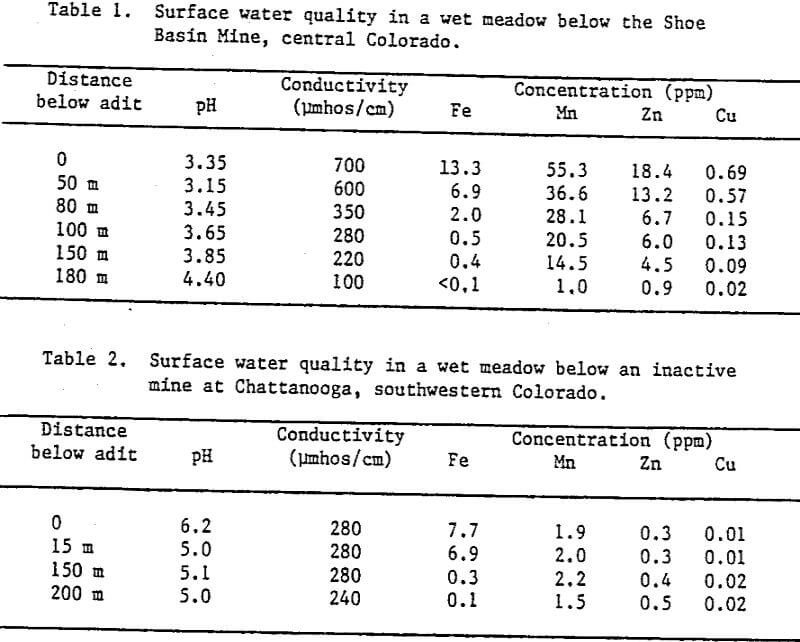
Surface water quality data from a wetland at the Chattanooga townsite shows a similar decrease in iron, but relatively little change in other metal concentrations. This site is located in the San Juan Mountains approximately five miles northwest of Silverton, Colorado, at an elevation of 3,346 m. Here, the initial pH was relatively high (6.2) where the discharge exits the adit at a flow of approximately 75 l/min, and dropped to 5.0 as it flowed through the wet meadow (Table 2). Both this and the Shoe Basin site have been abandoned for several decades. We do not know to what extent the wetland processes are in equilibrium with the metal load from the mines, but it is apparent that the vegetation, which was probably disturbed to some degree during the mining operations, is sufficiently acid- and metal-tolerant to recolonize both of these drainage-impacted areas. Improvement of water quality as mine discharge flows through wetland areas like the above, which have become naturally established, may be taken as an indication that wetlands may retain a long-term capability for removal of some metal species.