Table of Contents
We studied the problem of corundum formation on refractory linings in aluminum recycling furnaces. A laboratory test was developed for evaluating refractories in contact with molten recycled aluminum and identifying the mechanism of corundum formation. A test apparatus was designed and constructed to evaluate the performance of refractory samples. Corundum mechanism formation studies indicated that corundum formed on refractories when there is metal penetration. Once corundum forms, it acts as a wick allowing the molten aluminum to move to the surface where it is oxidized, thus causing further growth of the corundum. Phosphoric acid treatment or a spinel mortar coating prevented corundum formation on the 70- and 90-pct-Al2O3 refractories.
One objective of Bureau of Mines research is to reduce the need for critical minerals in high-performance refractories and extend the Nation’s reserves of domestic raw materials for refractories. As part of this objective, the Bureau, under a memorandum of agreement with Reynolds Metals Co., evaluated refractories in contact with recycled molten aluminum.
The increase in aluminum can scrap recycling by the aluminum industry has placed severe demands on refractories used by the industry. The recycling of aluminum requires the remelting of products containing oils, coatings, oxide layers, and other contaminants. Melting aluminum containing these types of contaminants requires larger quantities of salt flux to absorb impurities and protect the aluminum. Because of these contaminants and the increased production rates at aluminum recycling plants, more wear and corrosion of the refractories has resulted. The recycle furnaces are about 20 by 30 ft holding the molten metal at a depth of 4 ft. Along one side of the furnace, there are two large doors where the dross is skimmed off when it gets too thick. When the doors are opened for skimming, outside air enters the furnace. This air, plus the slight oxidizing atmosphere from the burners over a long period, causes another problem, which is the formation of corundum growths on the furnace walls. These corundum formations, when they are small, can sometimes be removed by the skimmers; however, the corundum that forms next to the doors or in the corners cannot be removed so easily and over a period of time gets quite large. These large corundum formations adhere tightly to the walls and when removed, take part of the walls with them.
This report describes the design of a laboratory furnace to evaluate refractories for use in recycling furnaces, and a study of the mechanism of corundum formation.
Materials and Equipment
A test apparatus was designed and constructed by the Bureau. It consisted of an electrically heated bottom loading type furnace with a 12 – by 12- by 9-in stainless steel shell in which up to 16 refractory samples could be cemented in place to form a refractory-lined cavity (fig. 1). In this cavity, up to 30 lb of molten aluminum alloy was maintained at a temperature of 1,550° F. The bottom of the cavity, made of a castable refractory, had a taphole for discharging the molten metal at the end of an experimental run. The refractories used in this study were all commercially available and are listed in table 1. Some of the samples were soaked in a ZrOCl2 solution—a technique based on a Bureau patent that resulted in improved high-temperature strength properties of alumina refractories. Some samples of the 70- and 90- pct-Al2O3 refractories were coated with a spinel mortar or treated with concentrated phosphoric acid prior to testing.
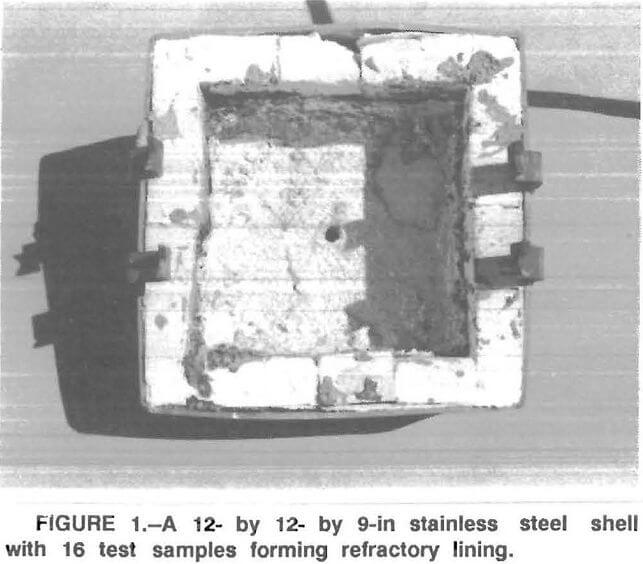
Test Procedure
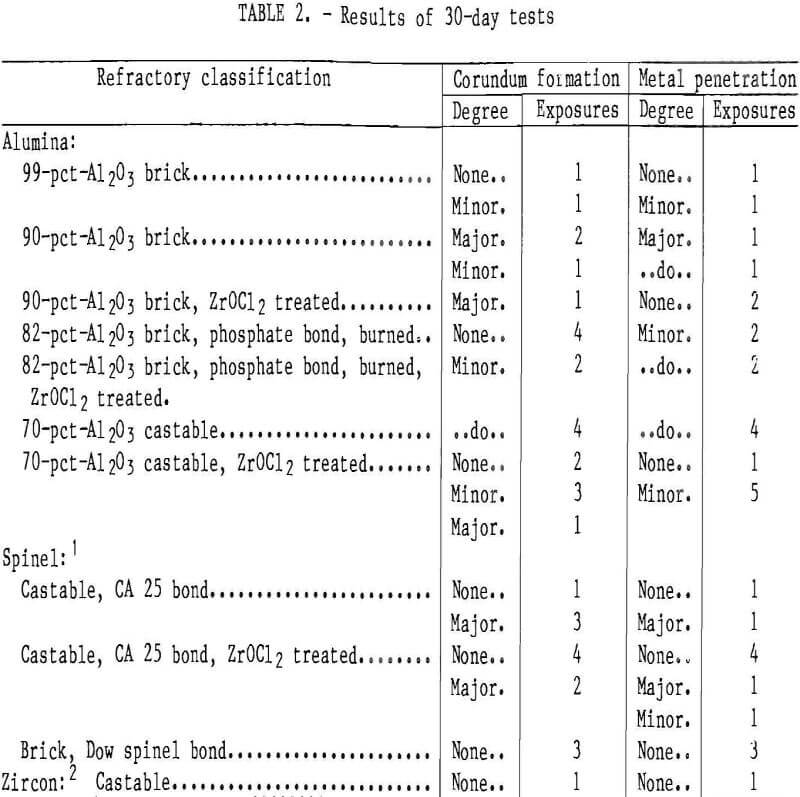
Six 30-day exposures were made using the testing apparatus. The test samples that lined the cavity holding the molten metal were exposed to the molten metal on one side, simulating conditions in the commercial furnace. In all tests, the molten metal was stirred and the oxidized aluminum that formed on the top was removed periodically. At the end of each test run, the molten metal was tapped, and the samples were allowed to cool to room temperature and removed for evaluation. The samples were sectioned to ob¬serve the extent of metal penetration. Table 2 lists refractories used in each test. Recycled aluminum alloy was used in all of these tests.
Based on the results of the 30-day tests it appeared that a period of 14 days would be adequate to satisfactorily evaluate the samples; so 14-day tests were conducted. In these tests, samples were suspended from a steel rod spanning the molten metal (fig. 2). This arrangement allowed the samples to be exposed to molten aluminum without being in contact with each other. This eliminated the problem observed in the 30-day tests where extensive development of corundum on a sample resulted in the corundum formation, in some cases, migrating to adjacent samples. The submerged part of the samples were exposed on all four- sides to the molten aluminum.
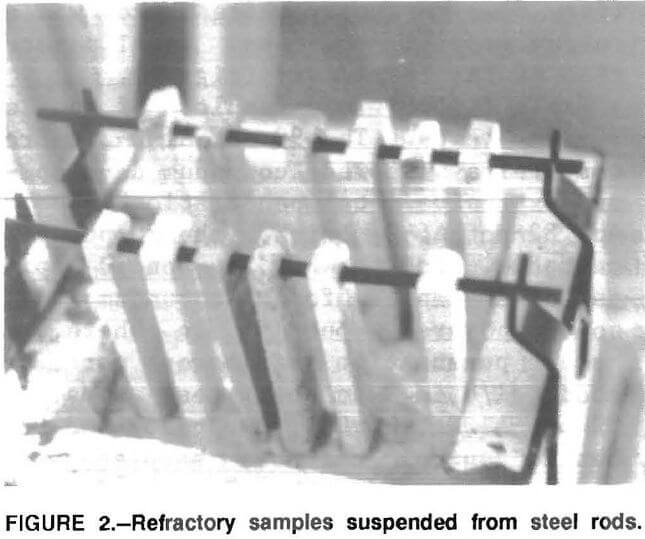
Results and Discussion
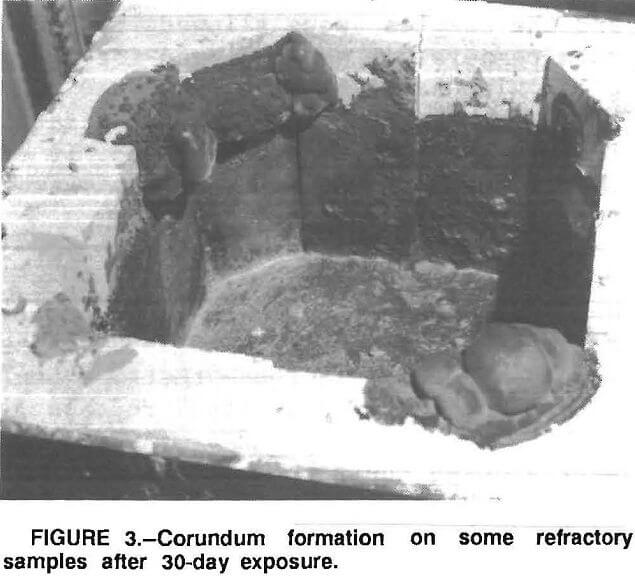
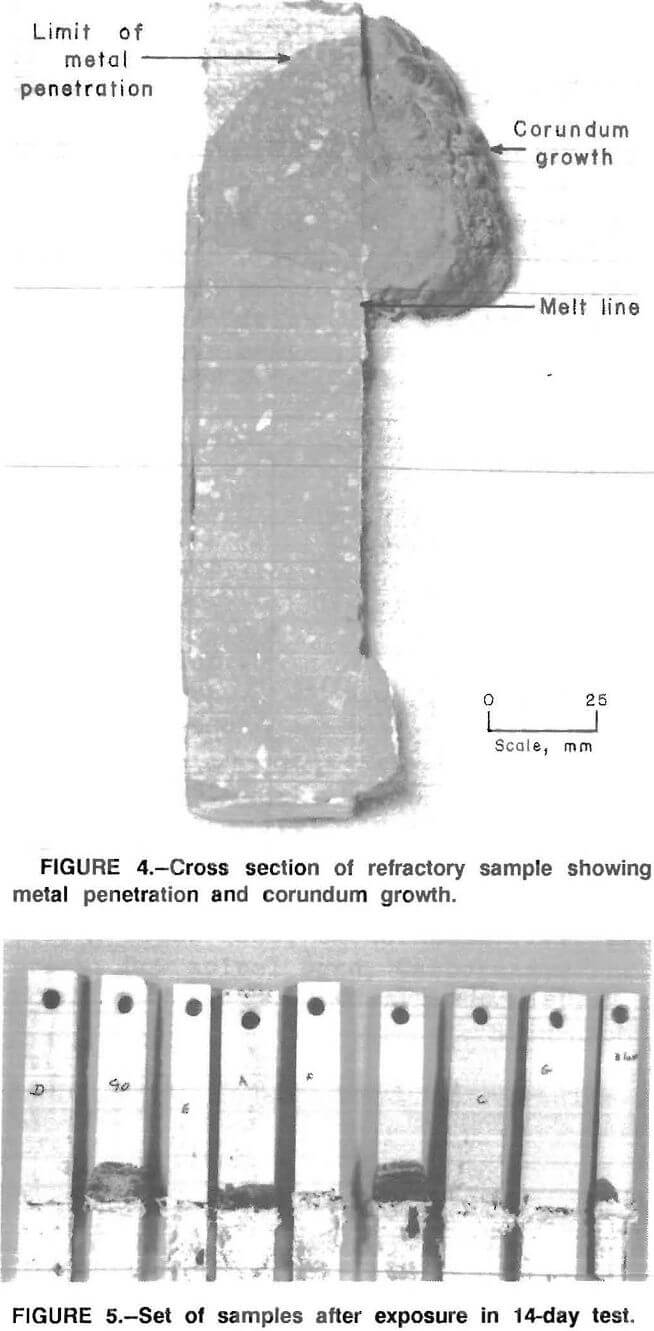
The results of the 30-day tests show that corundum developes on some of the refractories above the metal line similar to the corundum formation observed in commercial recycling aluminum furnaces (fig. 3). Further, where there was corundum growth, there was metal penetration of the refractory (fig. 4).
Table 2 lists the results of the six 30-day tests. The 90-pct-Al2O3 refractory always showed corundum development and extensive metal penetration. The 99- and 82-pct-Al2O3 brick, the 70-pct-Al2O3 castable, and the CA 25 bonded spinel castable samples formed corundum during some exposures, but not in others, and metal penetration was never more than about 1/8 in. In some test runs, the 82-pct-Al2O3, ZrOCl2 treated, and CA 25 bonded spinel castable samples showed some corundum growth. In these cases, however, a major development of corundum on an adjacent 90-pct-Al2O3 sample extended over to these samples. The spinel bonded brick and zircon castable samples never showed any corundum growth.
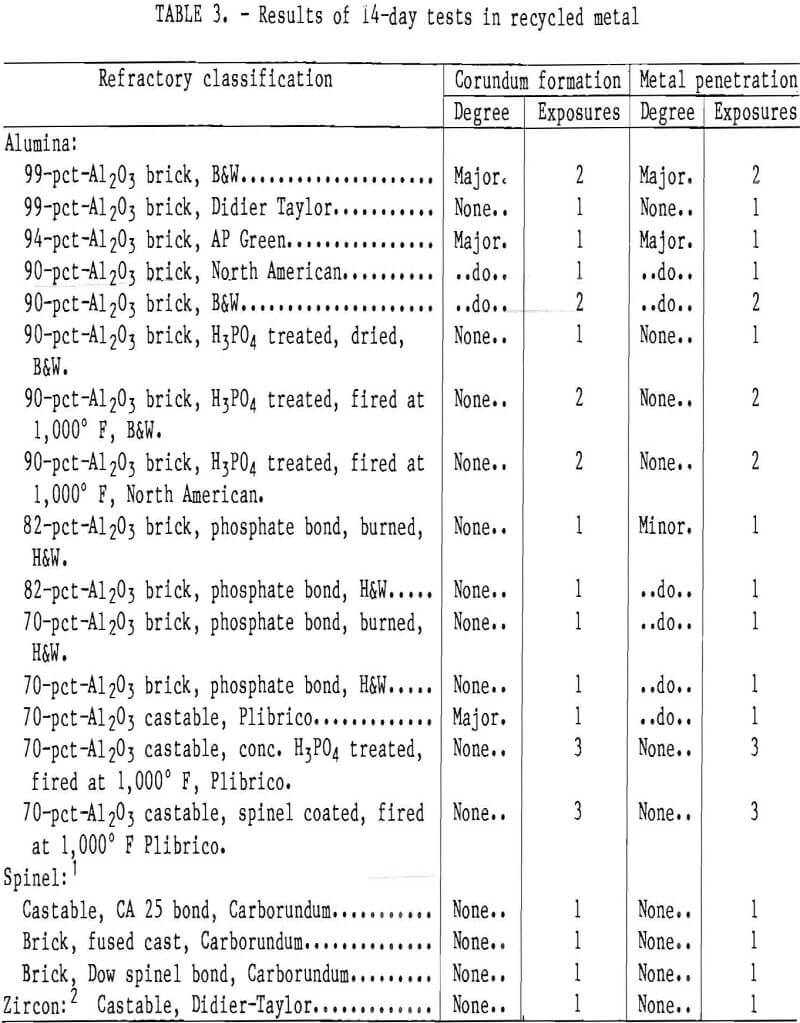
The 14-day test results confirmed the observations of the 30-day tests; i.e., corundum formed on some refractories and when metal penetration of the refractory occurred, corundum was formed. Table 3 shows the results of four 14-day tests where the refractories were exposed to recycled aluminum melts. Figure 5 shows a set of samples after one of the 14-day tests. The 90- and 94-pct-Al2O3 brick samples always developed corundum and had extensive metal penetration when exposed to the alloy melts. The 99- and 70-pct Al2O3 samples developed some corundum and showed slight metal penetration. The phosphate-bonded 70- and 82-pct-Al2O3 refractories, the zircon, and the spinel refractories all showed no corundum formation. The 70- and 90-pct-Al2O3 refractories that were treated with phosphoric acid or coated with spinel mortar and fired to 1,000° F showed no corundum development or metal penetration. Table 4 lists the results of exposures of refractory samples to a high-purity aluminum melt, a recycling alloy melt (0.6 to 1.0 Mg), and a high-magnesium (2.5 to 3.0 pct Mg) aluminum melt. No major differences in corundum formation or metal penetration were noted. Visual observations indicated, however, more metal penetration of samples by the high-purity aluminum melt. Also, when corundum developed on samples it appeared to develop faster when magnesium was present in the melts.
In summary, when corundum growths appeared, there was metal penetration of the refractory, but the formation of the corundum material on certain refractories in the 30-day tests occurred because these refractories were next to ones that developed major corundum growths. This problem was eliminated in the 14-day tests by having the samples separated. The results of these tests showed conclusively that corundum formed only when there was metal penetration of the sample.
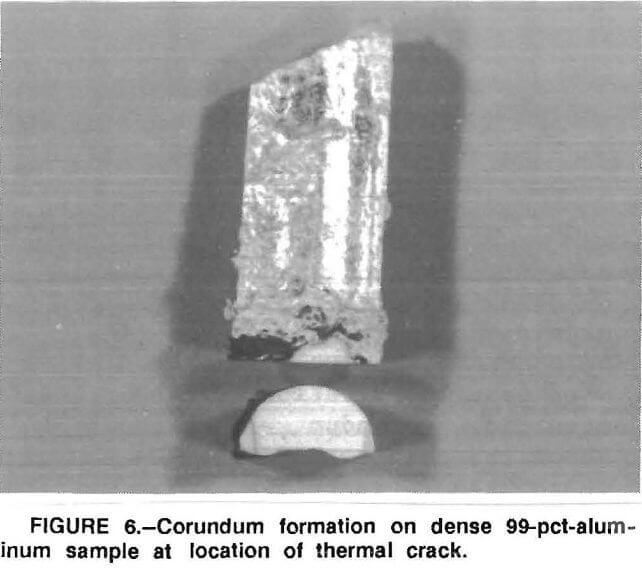
The test results suggest that the molten metal penetrates the porous refractory, migrates upward above the metal line to the refractory surface, then is exposed to an oxidizing atmosphere where corundum develops. This is dramatically demonstrated in a high-density 99-pct- Al2O3 sample where a thermal crack in the sample extended from below the metal line to above it. As shown in figure 6, corundum did form on this sample at the point where the crack exposed the molten aluminum to the furnace atmosphere. The presence of magnesium in the recycled
alloy metal seems to affect the rate of corundum growth after the growth starts. This agrees with results reported by the Lanxide Corp., Newark, DE in the formation of its “Lanxide” ceramics.
Corundum did not develop on the phosphate-bonded alumina or spinel refractories. This was also the case when the 70- and 90-pct-Al2O3 refractories were treated with a phosphoric acid solution or coated with a thin layer of spinel mortar (figs. 7-8). This may be due to
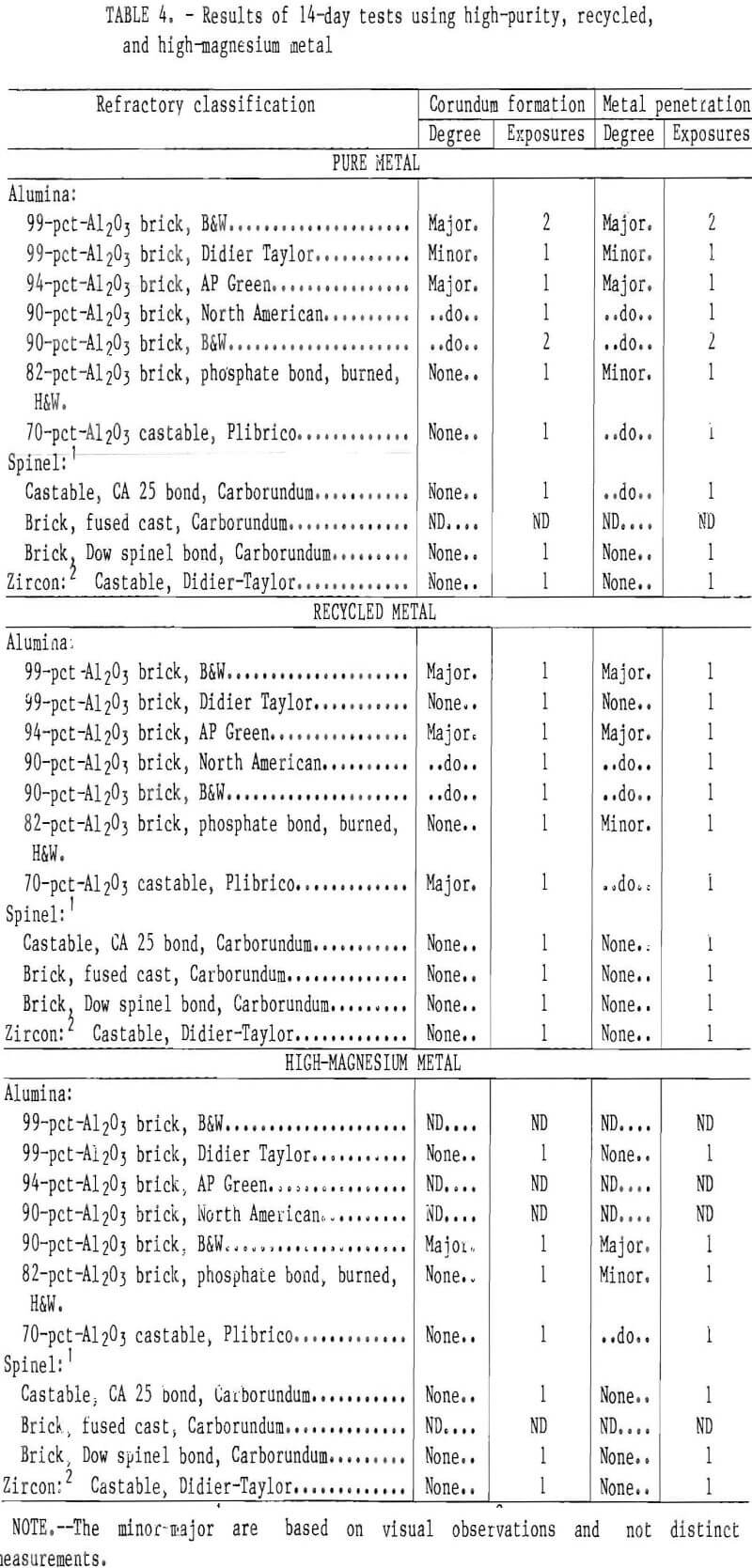
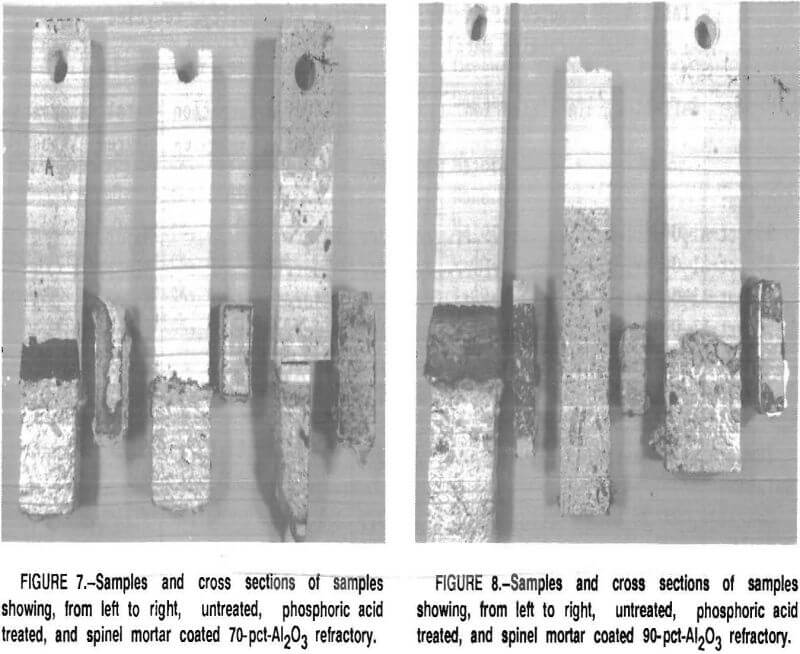
the refractory surface becoming less wet-table. It appears that a monolithic refractory furnace lining may need only a surface treatment or “wash” of phosphoric acid or spinel mortar In areas where corundum formation is a problem, i.e., in the corners of the furnace or around the loading ports. This treatment should be evaluated under industrial conditions in recycling furnaces.
Conclusions
Based on the study of corundum formation on refractories exposed to recycled aluminum melts the following conclusions can be made:
- A procedure was developed that duplicated the corundum formation in commercial aluminum recycling furnaces. This allowed the evaluation of refractories for use in these furnaces.
- Whenever corundum formed, there was some metal penetration of the refractory. The molten metal migrated through the refractory up above the metal line to the refractory surface where the melt oxidized and corundum formed. After the initial development of corundum, aluminum metal continues to be brought to its surface, through a wicking action, and additional corundum forms.
- Corundum always formed on the 90- to 94-pct-Al2O3 refractories evaluated in this study.
- Corundum did not develop on commercial phosphate-bonded 70- and 82-pct- Al2O3, zircon, or spinel refractories.
- Phosphoric acid treatment or a 1 spinel mortar coating prevented corundum formation on 70- or 90-pct-Al2O3 refractories.
