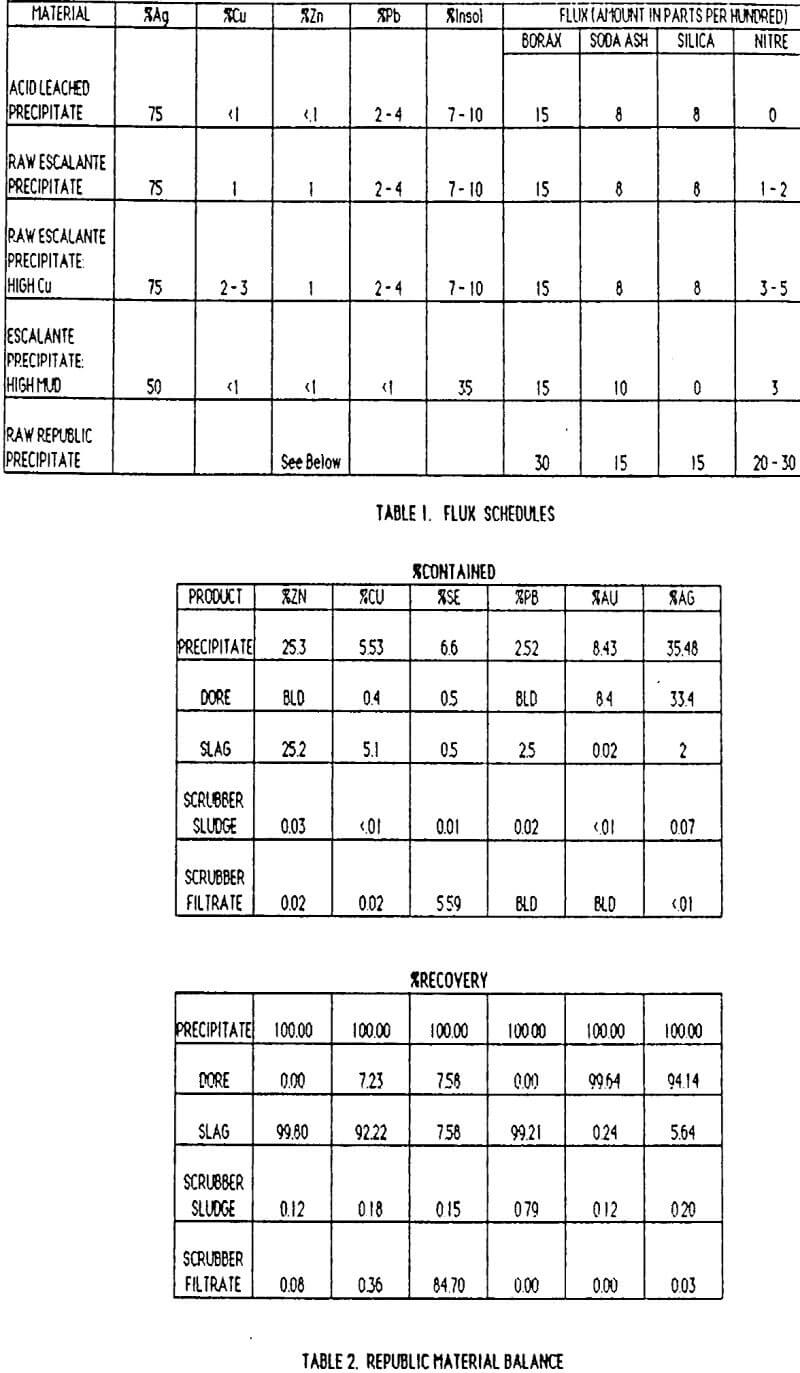
Table of Contents
Significant copper levels were anticipated in the precipitate due to cyanide soluble species in the ore. Original design called for a hydrochloric acid leach of precipitates to remove excess zinc and copper prior to fusion. It was thought that the use of hydrochloric acid would result in low silver losses because of the low solubility of silver chloride. Bench scale tests were run using hydrochloric acid and a variety of oxidizers. Leaching of copper and zinc was efficient with very little silver solubilization at a pH of 1.0 – 1.5 using an air sparge for oxidation.
An acid leach facility was designed and built for hydrochloric acid leaching. The first batch of high-copper precipitates was treated in the above manner. Leaching appeared to go as planned with satisfactory zinc and copper removal and essentially no silver dissolution. These precipitates were filtered, washed, fluxed, and loaded into the furnace for melting. A problem became apparent when a thick blue-green smoke was generated. The buttons obtained from this melt were a black graphlte-like material rather than dore’. Analysis showed this material to be fused silver chloride. During the leach, elemental silver had been converted to silver chloride. The probable chemical reactions are:
4Ag + 4H+ + 4Cl- + O2 = 4AgCl(s) + 2H2O
2Cu + O2 + 4H+ = 2Cu+2 + 2H2O
Zn + 2H+ = Zn+² + H2(g)
A solid to solid transformation had taken place producing a material unsuitable for fusion. An investigation commenced to determine:
a) A suitable leaching method to remove excess zinc and copper.
b) A method to recover values from the remaining hydrochloric acid leached precipitate.
Bench scale tests were performed using sulfuric acid and a variety of oxidizers. Efficient leaching of zinc and copper was obtained using sulfuric acid at a pH of 1.0 – 1.5 and an air sparge for oxidation. Oxidation-reduction potential (ORP) was monitored throughout the leach. Break-points (rapidly changing ORP) were noted and found indicative of the most significant reaction taking place at that stage. The following reactions tended to occur sequentially:
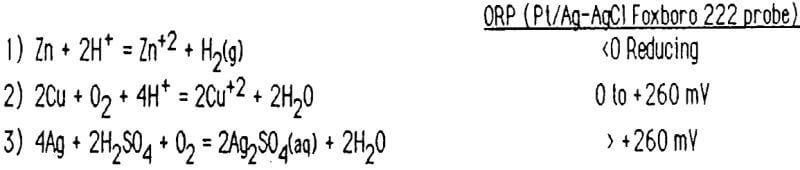
Equipment to continuously monitor the pH and ORP was installed in the acid leach vessel and this process has worked well for six years.
The precipitate which had been converted to silver chloride was reslurried, acidified with sulfuric acid, and reduced to elemental silver by adding zinc dust. It may also have been possible to achieve this reduction with a soda fusion.
In retrospect, the problem should have been anticipated. A more detailed analysis of the leached precipitate and/or larger scale fusion tests would have alerted us to the silver chloride problem.
Lead Contamination of Dore
Escalante ore contains lead species soluble In alkaline cyanide solutions. This is a mixed blessing. Lead, which reports to the precipitate, creates an exposure and impurity problem; however, it also has a beneficial effect in lowering zinc consumption and producing a permeable cake.
An investigation of lead solubilization revealed the following:
a) The lead solubility is very ore dependent and not necessarily related to the total lead content.
b) The solubilized lead species is colloidal in nature.
c) The solubility is pH dependent, increasing with alkalinity.
Lab tests revealed that lead removal could be accomplished by increasing the oxidizing power of our flux through soda ash reductions and/or the judicious addition of sodium nitrate. Dore’ with less than 1% lead was produced by reducing soda ash in the flux from 16 to 8 PPH (parts per hundred) of precipitate. In addition, the cyanide leach circuit pH was lowered from the 11.0-11.5 range to 10.5-11.0. This cut lead solubilization about in half.
Nitre Fusion
The latest process change has been the replacement of acid leaching with the judicious use of sodium nitrate in the flux. This change was tried during a refinery backlog, since a full day can be saved by skipping the acid leach. Additions of 1-2 PPH of sodium nitrate resulted in very high quality dore'(+ 98% silver), and the slag actually appeared cleaner. Plant tests were run to compare acid leaching prior to fusion with a direct fusion with 1 PPH sodium nitrate, A weeks precipitate production was split in half for parallel testing. The following results were obtained:

With a slag/dore ratio of about 0.5, acid leaching results in 75.2 kg (2417 tr. oz) silver per year higher slag loss than a direct fusion with 1 PPH sodium nitrate. An additional 24.3 kg (780 tr. oz) of silver were lost each year during the filtering of acid leached precipitate. The cost of reagents is about $4000/year less for a nitre fusion and at least $ 1000 /year in acid leach maintenance costs are saved. Overall, $27,000/year has been saved by eliminating the acid leach. Additional benefits of time saving and improved safety are also realized. Since the beginning of 1988 we have used a direct fusion with sodium nitrate successfully.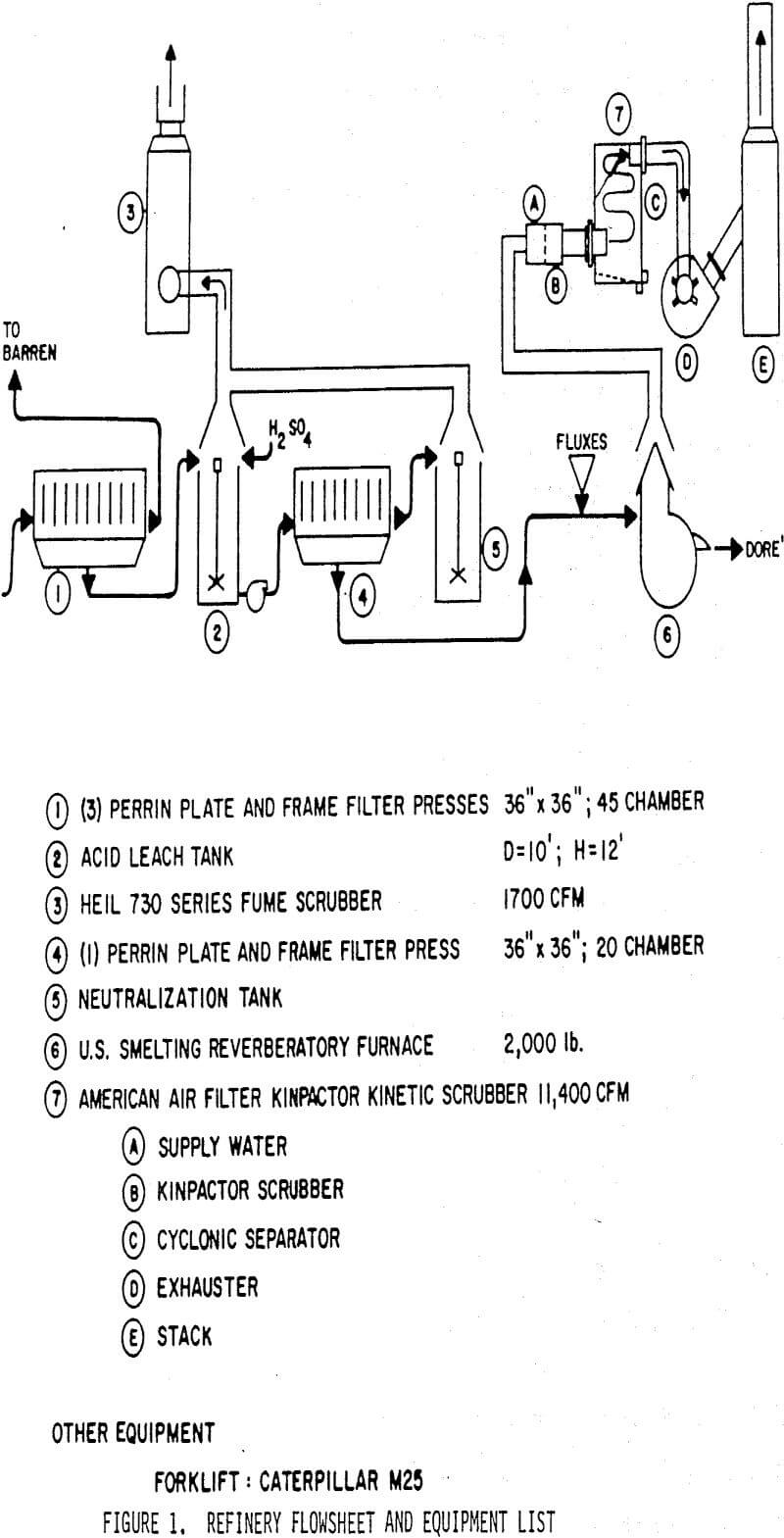
In defense of the decision to build the acid leach system, it must be noted that actual copper and zinc concentrations are much lower than those anticipated at the design stage. Early on, it was found that copper precipitation could be reduced substantially by maintaining a minimum on the barren solution of 1.7 ppm (0.05 oz/T) silver. When enough zinc is added to strip barren solutions much lower, appreciable quantities of copper are precipitated. If faced with a similar situation, one might design for the easy addition of acid leaching, but not install it initially.