Hydrogen peroxide (H2O2) oxidizes free and weakly-complexed cyanide in a one-step reaction to yield cyanate. Metals such as copper are precipitated simultaneously as hydroxides.
CN- + H2O2 → OCN- + H2O………………………………………………………….(1)
2 Cu(CN)3²- + 7H2O2 + 2OH- → 6OCN- + 2Cu(OH)2 + 6H2O…………………………………..(2)
The strongest metal-cyanide complexes commonly encountered in the effluents from gold and silver leaching operations are those of iron. These can now be removed in a second treatment step in which copper (II) ions are added to precipitate copper (II) ferrocyanide:
2Cu²+ 4 Fe(CN)6 4- → Cu2Fe(CN)6…………………………………………………(3)
These steps are shown in diagrammatic form in Figure 1, which also illustrates the relationship to the standard analytical methods.
It should be noted that the removal of copper (II) ferrocyanide by precipitation is only possible as part of a combination treatment, with H2O2 in the first step. This is because H2O2, unlike hypochlorite for instance, does not oxidize ferrocyanide to ferricyanide in alkaline solution. In fact, if any ferricyanide were present, this would be reduced to ferrocyanide with H2O2, thus enabling its precipitation. Excess hydrogen peroxide decomposes quickly to give water and oxygen:
2H2O2 → 2H2O + O2……………………………………………………………….(4)
The first plants to utilize this technology have already been installed at Canadian gold mills by Degussa. For the first time, a detoxification process is available which combines the environmental advantages of hydrogen peroxide and the ability to meet limits on total cyanide – even if ferrocyanide is present.
The procedure described above is capable of reducing concentrations of weak-acid dissociable cyanide to 0.1 ppm and total cyanide in most cases to less than 1 ppm. Although metals which were originally present as complex metal cyanides, are precipitated at the same time as the cyanide is oxidized, it can occur that some proportion of the dissolved metals is present ‘in some other complex form, such as an ammine complex. These metals can also be precipitated using an additional Degussa reagent called TMT 15. This is a 15% aqueous solution of the trisodium salt of trimercaptotriazine. TMT forms highly insoluble precipitates which can be removed easily from the wastewater before discharge, as in a polishing pond, for instance.
e.g. 3Cu²+ + 2TMT³- → Cu3TMT2……………………………………………………(5)
More information on TMT 15 and on the chemistry of cyanide detoxification is available in Degussa’s company literature.
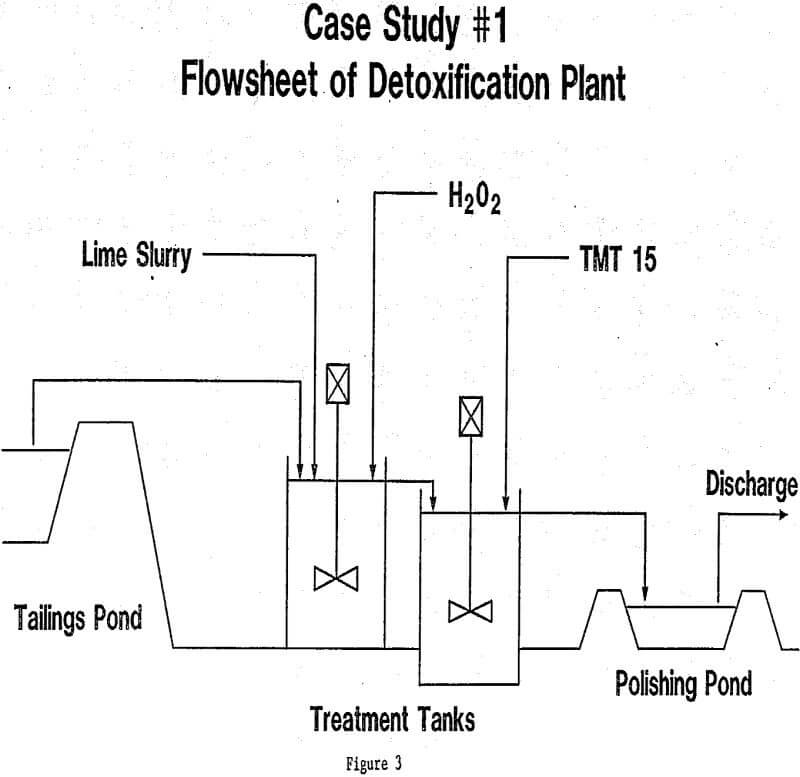
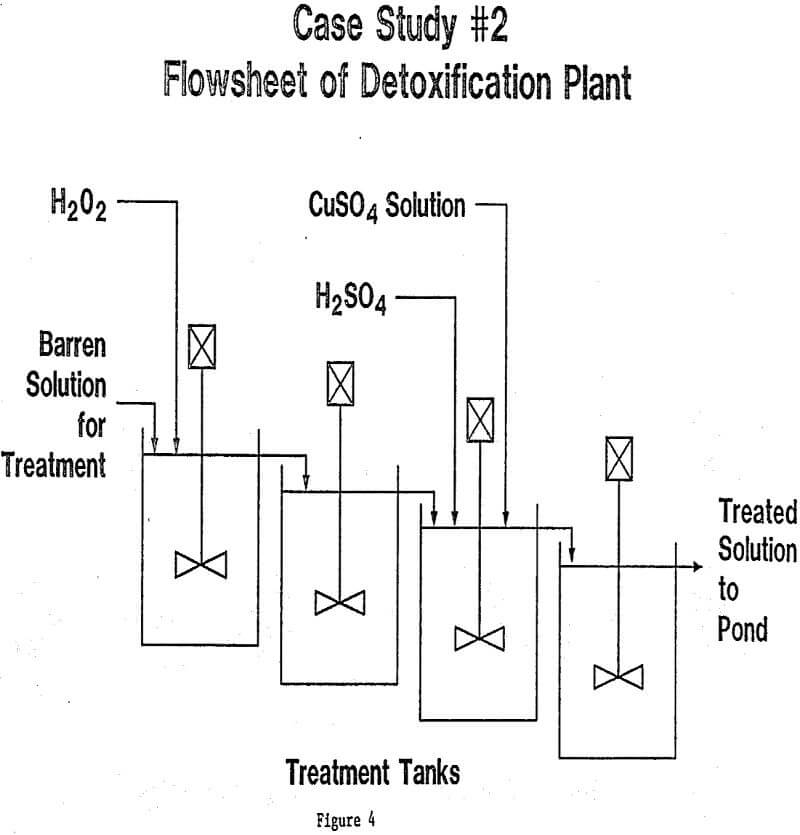
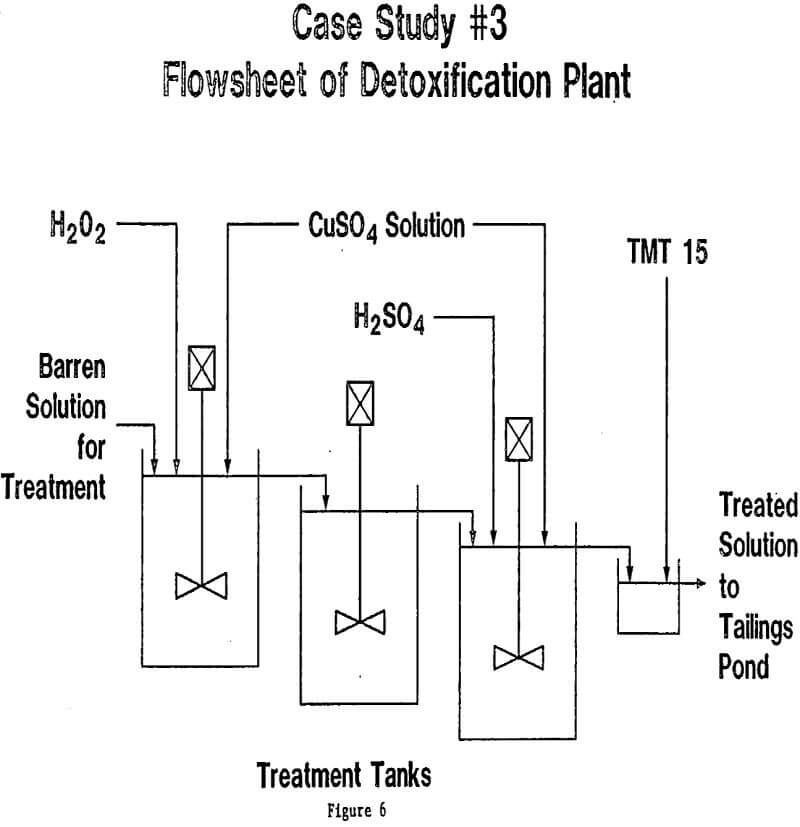
In each of the three cases described here, as well as the thirty or so cases that are currently under study in our laboratories, the final design for the treatment plant was arrived at after a long period of laboratory investigation, followed in some cases by pilot testwork and field optimization. It must be emphasized that waste water streams vary so widely in composition and characteristics that no one plant design can ever suit all cases. Hydrogen peroxide is, however, so flexible in its role as oxidizing agent, that it can be combined with other treatment steps in a wide variety of ways. Further examples might be cited:
- Removal of weakly-complexed cyanide with H2O2 followed by arsenic precipitation with ferric chloride. In this case H2O2 has the beneficial side effect of oxidizing arsenite to arsenate and improving the efficiency of the precipitation stage.
- Oxidation of cyanide combined with reduction of the chemical oxygen demand (COD). H2O2 has the considerable advantage over other oxidizing agents that it does not increase the content of dissolved solids in the water (TDS). Instead it provides a valuable source of dissolved oxygen, which then can be utilized by fish and other aquatic life.
- Oxidation of sulfur compounds in low oxidation states. Substances such as sulfide, thiosulfate, and sulfite are of concern at certain sites. These can be oxidized to sulfate by H2O2 without adding to the TDS content.
The capital and operating costs of treatment plants can vary quite widely. This is true whichever process is used. The main items of capital equipment required for treatment with H2O2 are the agitated tanks, which can be of simple design. The storage and dosage equipment for H2O2 can be leased if preferred.
The main contributor to the operating costs is the consumption of hydrogen peroxide. This can be reduced in a number of ways, such as automating the dosage or by designing the mill in such a way that the effluent treatment costs are minimized. Up to now, the effluent treatment has usually been regarded as a costly appendage to the gold mill, whereas an integrated concept would often lead to considerable savings in operating costs.
Hydrogen peroxide has been used widely in industry for cyanide detoxification, for example. The process has the advantage over techniques that use chlorine or sulfur dioxide in that no foreign ions are introduced into process solutions, unless a catalyst is used, and the kinetics of oxidation are sufficiently fast that effective oxidation can generally be achieved in a few minutes. However, detoxification to the low-residual cyanide concentrations usually required for an effluent discharge is costly. Hydrogen peroxide oxidizes free cyanide to cyanate as follows:![]()
The efficiency of hydrogen peroxide cyanide destruction has been demonstrated repeatedly, and it is well known that solutions containing 500 mg/L WAD cyanide, or more, can be reduced to <2 mg/L within practical time scales, for example, 1 to 2 hr, by the addition of 75 to 125 mg/L H2O2. Final effluents containing <0.1 mg/L CN- can be produced at higher peroxide dosages, but the economics of this are generally unfavorable.
Hydrogen peroxide consumption is estimated to be approximately 3 kg H2O2/kg CN–. However, other species present in the solution or slurry may compete with cyanide for peroxide and may increase consumption by direct reduction and/or by catalytic action.
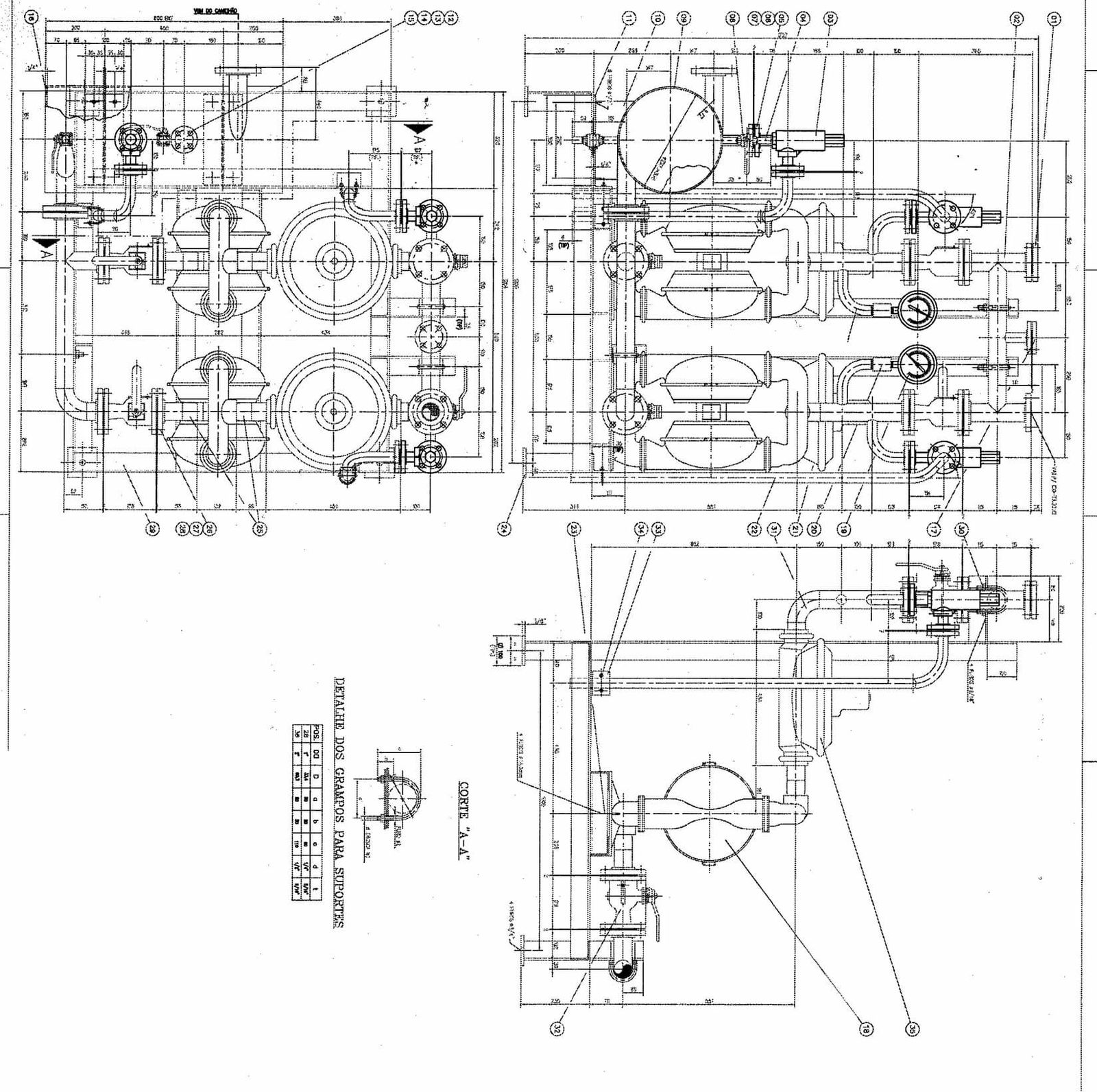
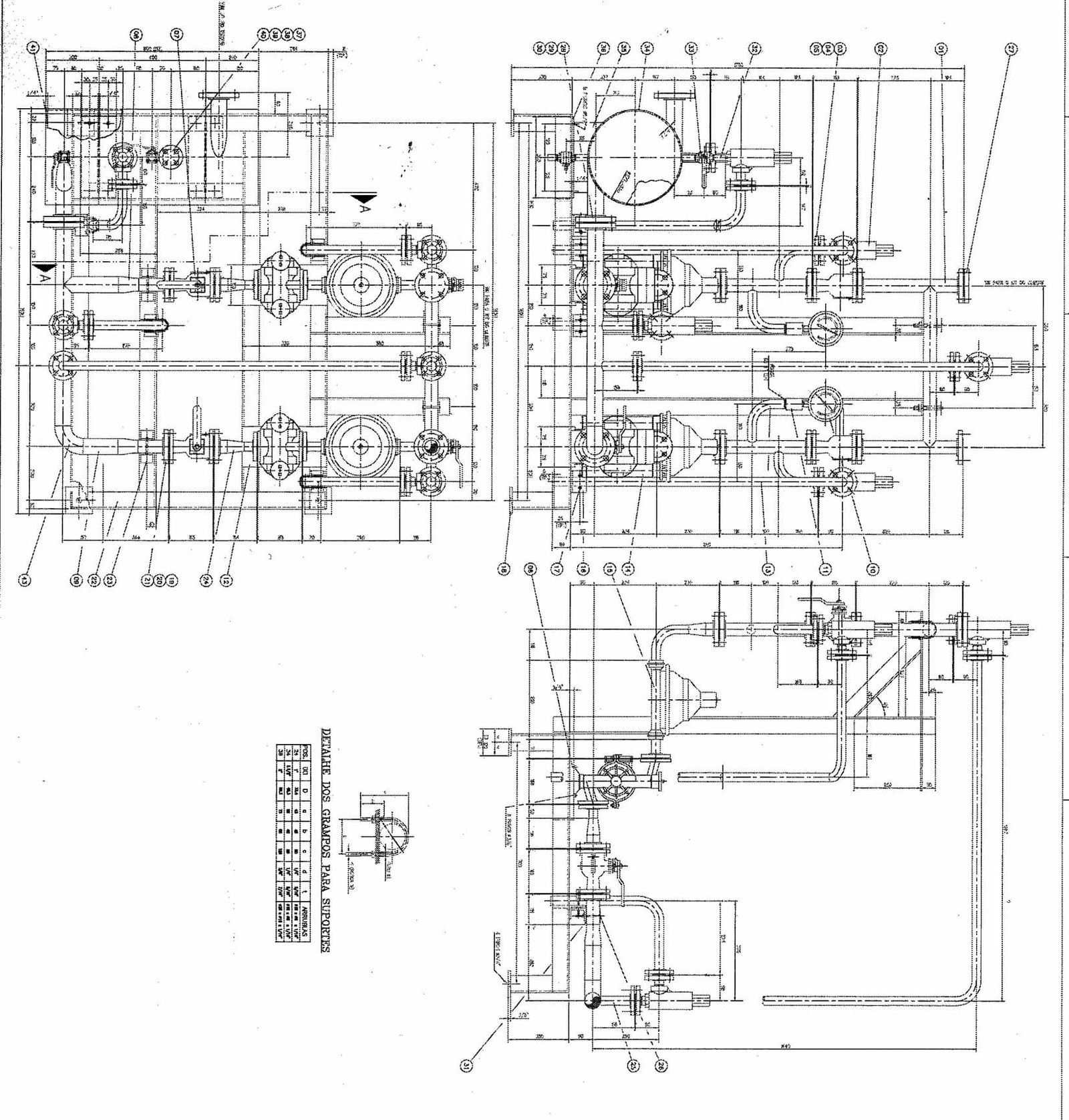
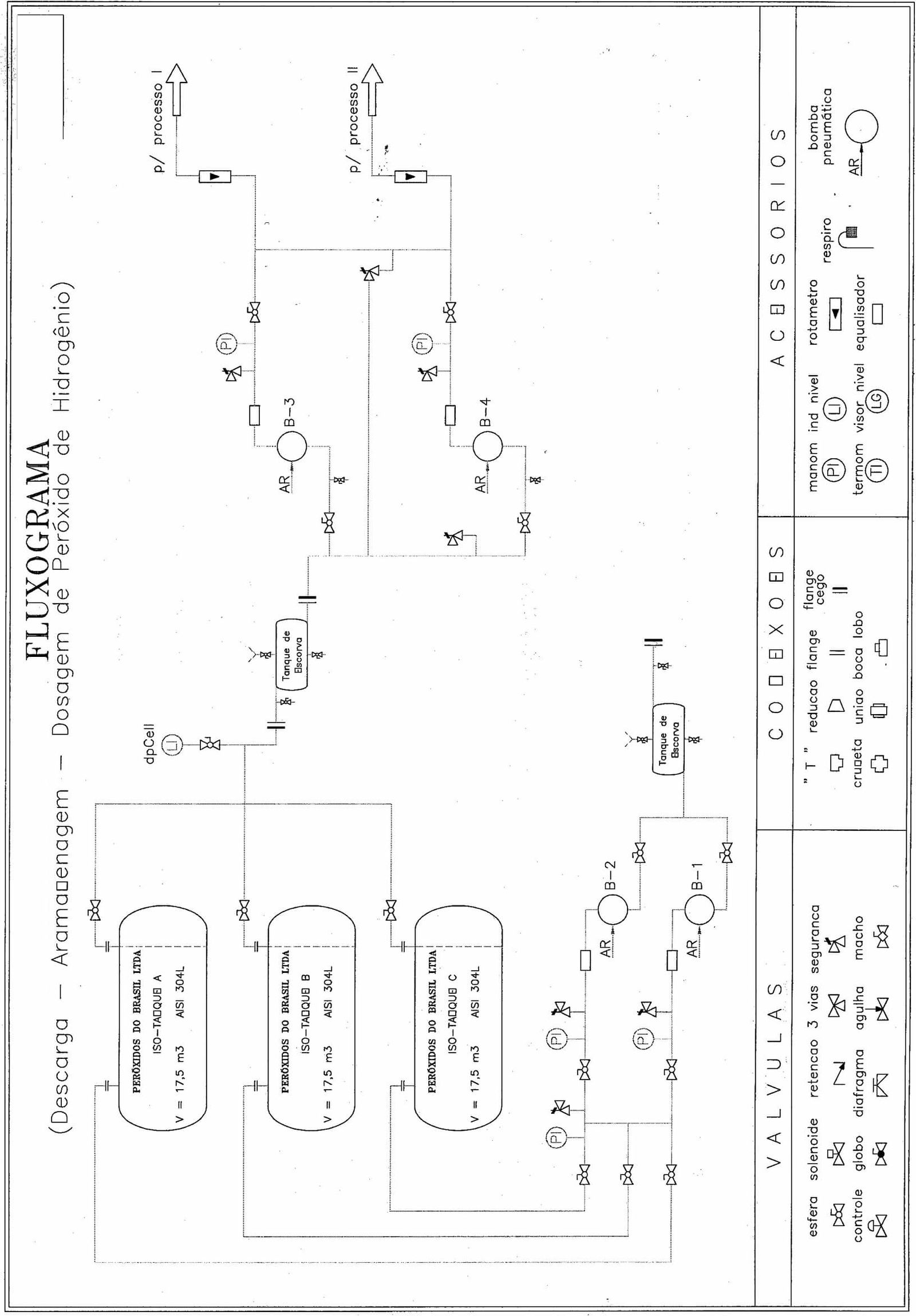
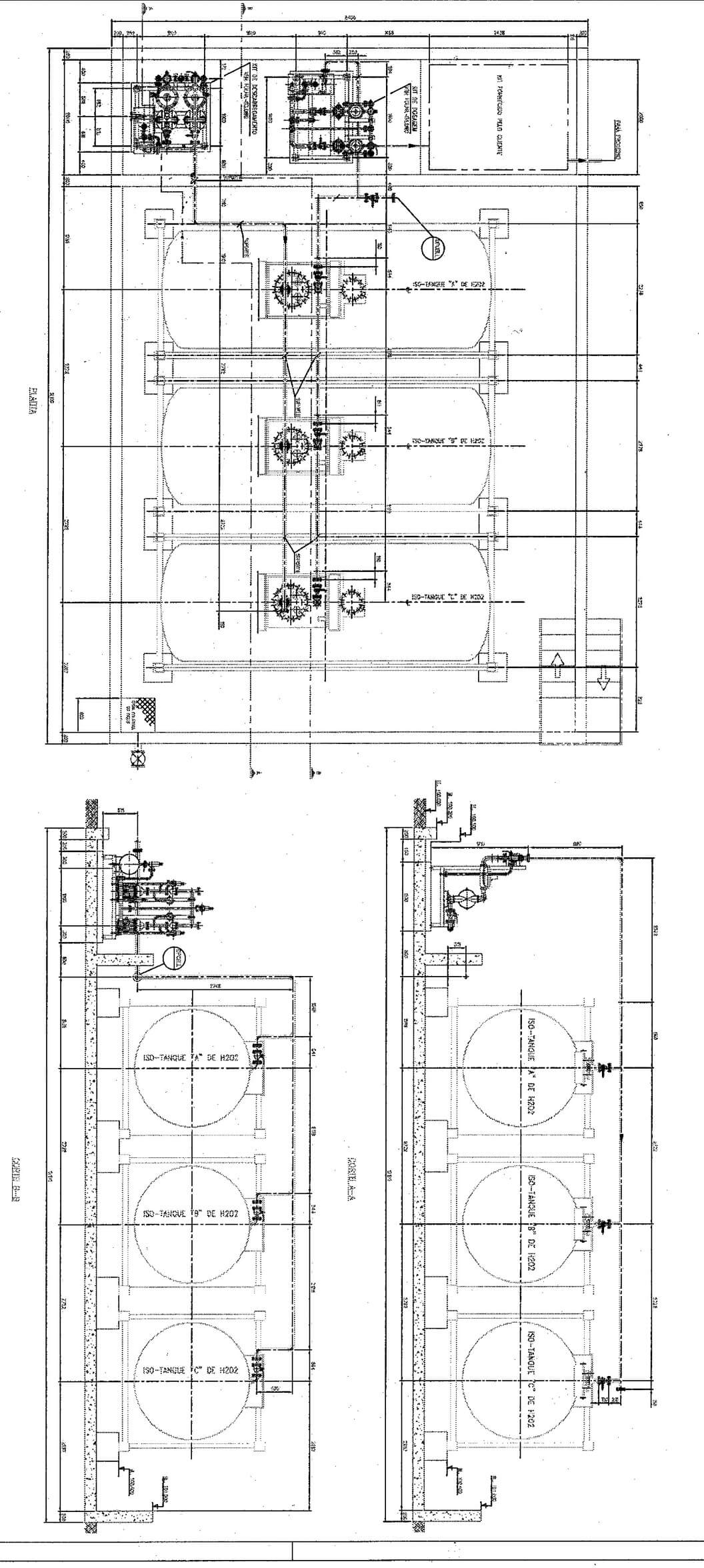
 |  |
 |  |