Table of Contents
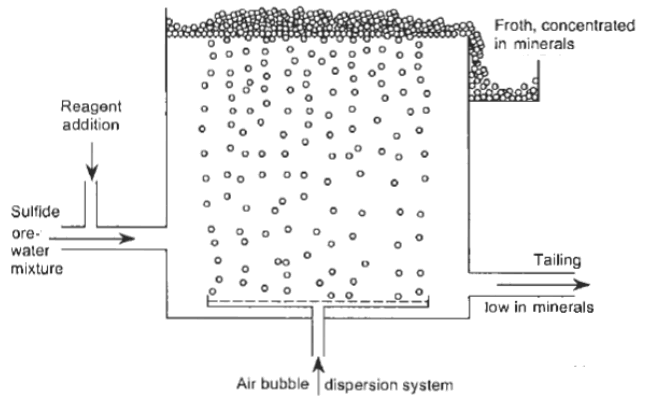
The Froth Flotation Process is about taking advantage of the natural hydrophobicity of liberated (well ground) minerals/metals and making/playing on making them hydrophobic (water-repel) individually to carefully separate them from one another and the slurry they are in. For this purpose we use chemicals/reagents:
- Frothers (MIBC) is what allows the formation of air bubbles. Think of frother as soap in your bathtub.
- Collectors (5100, 3418, Xanthate) are used as the base to making metals hydrophobic (water repellent) and allows it to attached to air bubbles. Think of collectors wax (water-repellent) on car paint that allows the water pearl away.
- CuSO4 is the “activator” used to make Zn (sphalerite) ready to be covered by Collector. In the eyes of the Collector, activated Zinc is Copper. Think of CuSO4 as epoxy primer on raw metal before you paint it.
- pH Modifiers (MBS , Lime) are used to intensify or reduce collector’s water-repellent effect on mineral surfaces. This allows Flotation Collectors to act selectively towards certain minerals. Think of pH as the temperature of ambient air at which paint is applied to metal. Some paint work (stick) best to metals (than others) depending on certain temperature.
ASK QUESTION AND DISCUSS FLOTATION HERE
FROTH FLOTATION
“So many variables influence flotation that it will be long before every one of them can be investigated and its influence on the process determined.”
The froth flotation process was patented by E. L.Sulman, H. F. K. Pickard, and John Ballot in 1906, 19 years after the first cyanide process patents of MacArthur and the Forests. It was the result of the intelligent recognition of a remarkable phenomenon which occurred while they were experimenting with the Cattermole process. This was the beginning. When it became clear that froth flotation could save the ‘extremely fine free mineral’ in the slime, with a higher recovery than even gravity concentration could make under the most favorable conditions, such as slime-free pulp, froth flotation forged ahead to revolutionize the nonferrous mining industry. The principles of froth flotation are a complex combination of the laws of surface chemistry, colloidal chemistry, crystallography, and physics, which even after 50 years are not clearly understood. Its results are obtained by specific chemical reagents and the control of chemical conditions. It not only concentrates given minerals but also separates minerals which previously were inseparable by gravity concentration.
This new process, flotation, whose basic principles were not understood in the early days, was given to metallurgists and mill men to operate. Their previous experience gave them little guidance for overcoming the serious difficulties which they encountered. Few of them knew organic chemistry. Those in charge of flotation rarely had flotation laboratories. Flotation research was done by ‘cut and try’ and empirical methods. The mining industry had no well equipped research laboratories manned by scientific teams.
Froth Flotation Handbook
Froth flotation, as pointed out previously, was a part of the evolution of milling during the first quarter of the 20th century—a period during which the progress of milling was greater than in all of its previous history. It marks the passing of the stamp battery, after 400 years service to the mining industry, and the beginning of grinding with rod mills, ball mills, and tube mills without which neither the cyanide process nor the froth flotation process would have reached full realization. More than all of these, it was the time when custom and tradition were replaced by technical knowledge and technical control.
This volume, then, is dedicated to those men who, with limited means, made froth flotation what it is today. It is designed to record the impact of this great ore treatment development on the mining industry both present and future.
“The single most important method used for the recovery and upgrading of sulfide ores”, that’s how G. J. Jameson described the froth flotation process in 1992. And it’s true: this process, used in several processing industries, is able to selectively separate hydrophobic from hydrophilic materials, by taking advantage of the different categories of hydrophobicity that are increased by using surfactants and wetting agents during the process also applied to wastewater treatment or paper recycling.

Also see How froth flotation works.
Learn How to evaluate a flotation circuit.
The mining field wouldn’t be the same without this innovation, considered one of the greatest technologies applied to the industry in the twentieth century. Its consequent development boosted the recovery of valuable minerals like copper, for instance. Our world, full of copper wires used for electrical conduction and electrical motors, wouldn’t be the same without this innovative process.
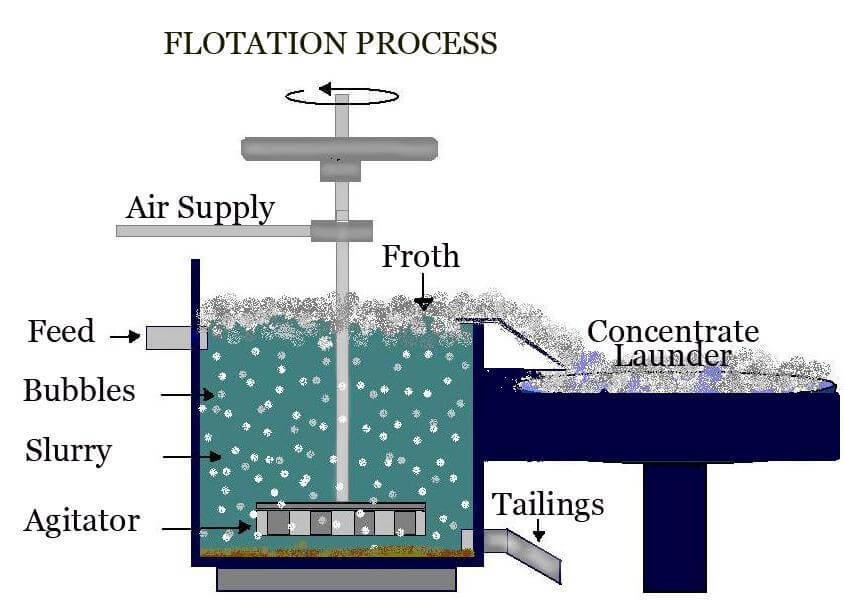
During the froth flotation process, occurs the separation of several types of sulfides, carbonates and oxides, prior to further refinement. Phosphates and coal can also be purified by flotation technology.
During the process, four things happen:
- Reagent conditioning happens in order to achieve hydrophobic surface charges on the desired particles
- Collection and upward transport by bubbles in contact with air or nitrogen
- A stable froth formates on the surface of the flotation cell
- There’s a separation of the mineral laden froth from the bath
The flotation process has three stages:
- Roughing
- Cleaning
- Scavenging
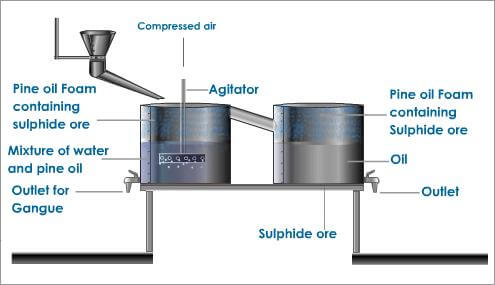
Flotation can be performed by different types of machines, in rectangular or cylindrical mechanically agitated cells or tanks, columns, a Jameson Flotation Cell or deinking flotation machines. The mechanical cells are based in a large mixer and diffuser mechanism that can be found at the bottom of the mixing tank and introduces air, providing a mixing action. The flotation columns use air spargers to generate air at the bottom of a tall column, while introducing slurry above and generating a mixing action, as well.
Mechanical cells usually have a higher throughput rate, but end up producing lower quality material, while flotation columns work the other way around, with a lower throughput rate but higher quality material. The Jameson cell just combines the slurry with air in a downcomer: then, a high shear creates the turbulent conditions required for bubble particle contacting.
Advantages of froth flotation: first of all, almost all minerals can be separated by this process. Then, the surface properties can be controlled and altered by the flotation reagent. Finally, this technique is highly appropriate for the separation of sulfide minerals.
Disadvantages of the flotation process: the method is quite complex and expensive, besides being influenced by the slime.
The process of froth flotation usually involves a series of steps:
- the preparation of appropriate particle sizes of liberated components in the mixture of solids to be separated;
- the creation of conditions favourable for the adherence of one or more components in the mixture of solids to attach to air bubbles; and
- the formation of a stable froth containing one or more components existing on the surface of the agitated mixture of particles (the pulp) which can be removed (recovered).
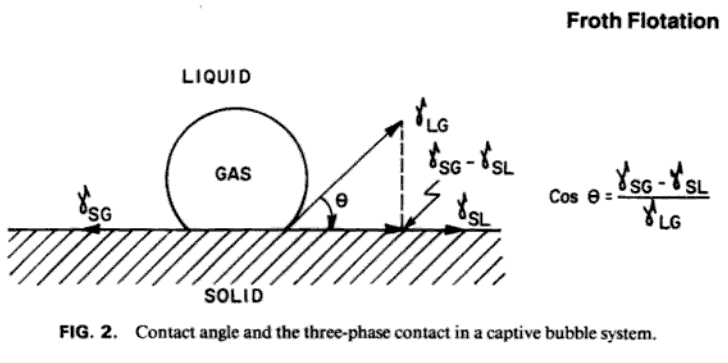
Bubble Contact Angle Froth Flotation
How Flotation Chemicals are Used
To help towards an understanding of the reasons for the employment of specific types of reagents and of the methods of using them, an outline of the principal theoretical factors which govern their application may be of service. For a full discussion of the theory of flotation the various papers and text-books which deal with this aspect should be consulted.
The physical phenomena involved in the flotation of minerals, those, for example, of liquid and solid surface-tensions, interfacial tension, adsorption, flocculation, and deflocculation, are the manifestations or effects of the surface-energies possessed by all liquids and solids in varying degree. These, in turn, arise from the attractions which exist between the interior molecules of every substance and are responsible for their distinctive properties—form, fluidity, cohesion, hardness, and so on. It follows, therefore, that every substance must exhibit some degree of surface-energy.
Functions of Flotation Reagents
All the solids normally present in an ore— i.e., metallic, non-metallic, and rock-forming minerals—have their particular contact-angle and hysteresis values and therefore tend to be wetted in varying degrees in accordance with such values. These differences, however, are not usually sufficient to allow of the effective separation of the mineral and gangue constituents from each other. It is the function of the flotation reagents employed to accentuate or magnify these differences to a degree which renders separation by flotation practicable. Some reagents (modifiers) are added with the object of decreasing the contact-angle and so increasing the degree of wetting of the unwanted particles, which are usually more prone to become wetted than the wanted minerals. Others (promoters) are added to increase the tendency toward “non-wetting” shown by the valuable minerals by coating them with a film of yet higher contact-angle value. Such films are said to be “ adsorbed ” in respect of the water.
In this connection reference to Fig. 28 will indicate that a reagent which decreases the surface-tension of water tends thereby to increase wetting of the solid, since, if the value of S1 and therefore of its horizontal component, is lessened, the water-edge, as at P, will tend to extend over the solid surface, making therewith a smaller contact-angle.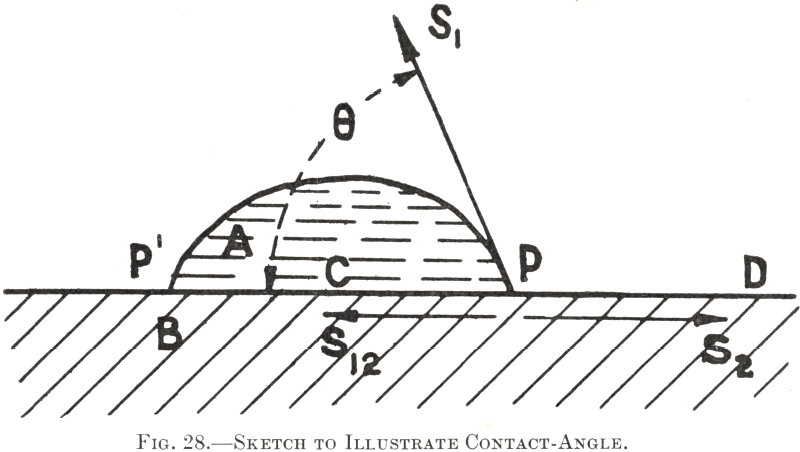
The reagents added to promote the separation of the wanted minerals by increasing the water/solid contact-angle consist of substances whose molecules or minute suspensions have a markedly lower attraction for water molecules than the latter exert between themselves. Finely divided oil emulsions in water, dissolved xanthates, and other “promoters” are typical of such reagents. Substances of such nature, when dissolved in or disseminated through water, are pre-eminently “adsorbed”, or thrust towards the water boundaries, where the intra-molecular attractions are less uniformly balanced. Normally, this would occur at the free or air/water surface. In a pulp, however, from which air surfaces are absent, but in which mineral particles are suspended, the same thing takes place at the water/solid boundaries, adsorption being most pronounced at those faces where the interfacial tension is greatest— viz., those with the highest contact-angle value and lowest adhesion for water. The minute particles of oil or xanthate molecules are thus virtually thrust into adherence with the more floatable solids, whose surfaces they therefore film, increasing the contact-angles to their own high values and so rendering the solid more floatable. Experimental work indicates that the film so formed is of the order of one molecule in thickness.
Adsorption can be both positive and negative. Substances whose molecules have less attraction for water than the water molecules have for each other are concentrated at the water boundaries as explained in the foregoing paragraph ; this is termed positive adsorption, but substances whose molecules have a greater attraction for water molecules than the latter have for each other will tend to be dragged away from the surface layers, at which their concentration thus becomes less than in the interior of the liquid ; this is negative adsorption. Substances that are negatively adsorbed are those which tend to form chemical compounds or definite hydrates with water, such as sulphuric acid. In froth flotation we are concerned more with positive than with negative adsorption.
In some cases a chemical reaction between the solid and the reagent occurs at the interface ; for instance, in the activation of sphalerite by copper sulphate a film of copper sulphide is deposited on the mineral following adsorption of the copper salt at its surface. In many cases there is no evidence of any chemical change, but, whether chemical action takes place or not, there is no doubt that the filming of the mineral is due primarily to the adsorption property of the liquid itself, by virtue of which the promoting reagent dissolved or suspended in it is concentrated at the interface.
The chemical action of flotation reagents has been and still is the subject of a great deal of research work, which is bringing the various theories into common agreement, but there are still too many doubtful points and unexplained phenomena to make a simple explanation possible in these pages.
Classification of Flotation Reagents
The foregoing paragraphs can be summarized by stating that the reagents employed in froth flotation can be classified into three general groups, comprising “frothers”, “promoters”, and “modifiers”, respectively, the purposes of each class being as follows :—
- The function of ” frothing ” reagents is to permit of the dissemination throughout the pulp of a cloud of small air-bells, which do not coalesce whilst in the body of the liquid, and also to stabilize the bubbles formed when they rise to the surface, so that they remain there without bursting as a more or less permanent froth. In other words, frothers reduce the surface-tension of the water by supplying a small amount of contaminant adsorbed at the air/water surfaces. The frothing reagent, however, requires choice and limitation in amount. The surface-tension of the water must not be reduced too much nor the contamination of its surfaces reach too high a concentration, otherwise the water layers surrounding the air-bells will not possess sufficient strain-energy to retain mineral particles. Soap, saponine, and other substances which give rise to intense frothing effects, cannot, as a rule, be employed alone, since they reduce the surface energy of the water to so low a value that insufficient remains to be utilized for mineral flotation ; the air/water froths so formed are barren and useless.
- The function of “promoting” reagents and “collecting” oils is to accentuate the property of the faces of the wanted minerals to become “non-wetted” by adsorption thereon of a film having a still higher contact-angle value than that shown by the minerals alone, and thus to increase their floatability.
- The function of “ gangue modifying ” reagents, or “ modifiers ”, is to accentuate the property of the faces of the rock or other unwanted gangue particles to become wetted by still further reducing the value of the contact-angles that they form with water, and so to decrease their floatability.
The nomenclature adopted is that which has grown up in practice. It is perhaps not scientifically exact, but it sufficiently indicates the purposes for which the reagents are employed.
The operation of flotation is not always confined to the separation of the valuable constituents of an ore in a single concentrate from a gangue composed of rock-forming minerals. It often happens that two classes of floatable minerals are present, of which only one is required. The process of floating one class in preference to another is termed “ selective ” or “ preferential flotation ”, the former being perhaps the better term to use. When both classes of minerals are required in separate concentrates, the process by which first one and then the other is floated is often called “ differential flotation ”, but in modern practice the operation is described as “ two-stage selective flotation ”.
Selective flotation has, therefore, given rise to two other classes of reagents, each of which may be regarded as falling within one of the classes already mentioned. They are known as “ depressing ” and “ activating ” reagents.
- A depressing reagent, or “ depressor ”, is a type of modifier whose function is to render unfloatable normally floatable minerals which are not wanted in the particular concentrate required.
- An activating reagent, or “ activator ”, belongs to the promoting class, and is employed to render floatable minerals which were originally insufficiently so or have been sunk by the use of a depressor.
The use of these reagents has been extended in recent years to three- stage selective flotation. For example, ores containing the sulphide minerals of lead, zinc, and iron, can be treated to yield three successive concentrates, wherein each class of minerals is recovered separately more or less uncontaminated by the others.
Flotation Processing Costs
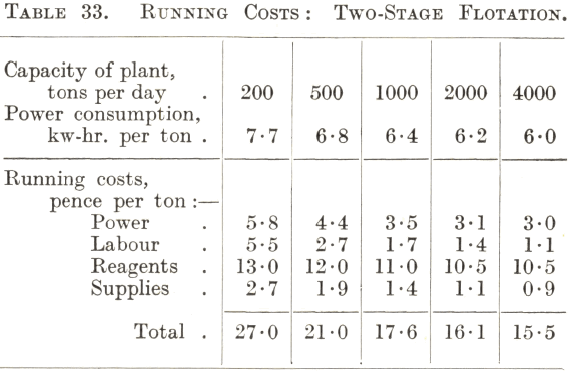
Although the flotation of the commoner ores, notably those containing copper and lead-zinc minerals, has become standardized to some extent, there is nevertheless considerable variation in the amount and nature of the reagents required for their treatment. For this reason the running costs of the flotation section of a plant are somewhat difficult to predict accurately without some test data as a basis, more especially as the cost of reagents is usually the largest item. Tables 32 and 33 can therefore only be regarded as approximations. Table 32 gives the cost of the straightforward treatment in air-lift machines of a simple ore such as one containing easily floated sulphide copper minerals, and Table 33 that of the two-stage selective flotation of a lead-zinc or similar complex ore.
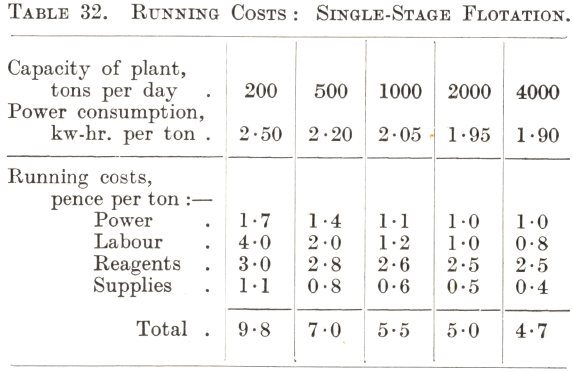
From Table 32 it will be seen that the reagent charge is likely to be the largest item even in the flotation of an ore that is comparatively easy to treat, except in the case of a very small plant, when the labour charge may exceed it. At one time the power consumption in the flotation section was as expensive an item as that of the reagents, but the development of the modern types of air-lift and pneumatic machines has made great economies possible in expenditure under this heading. As a rule Callow-Maclntosh machines require less power than those of the air-lift type to give the same results, while subaeration machines can seldom compete with either in the flotation of simple ores, although improvements in their design in recent years have resulted in considerable reductions in the power needed to drive them. It should be noted that the power costs given in the table include pumping the pulp a short distance to the flotation machines, as would be necessary in an installation built on a flat site, and the elevation of the rougher and scavenger concentrates as in circuits such as Nos. 9 and 10.
The power costs decrease with increasing tonnage because of the greater economy of larger units and the lower price of power when produced on a large scale. The cost in respect of reagents and supplies also decreases as the size of the plant increases, due to better control and organization and to lower first cost and freight rates of supplies when purchased in bulk. The great disadvantage of a small installation lies in the high labour cost. This, however, shows a rapid reduction with increase of tonnage up to 1,000 tons per day, the reason being that with modern methods a flotation section handling this tonnage requires few more operators than one designed for only 200 tons per day. For installations of greater capacity the decrease is comparatively slight, since the plant then generally consists of parallel 1,000-ton units, each one requiring the same operating force ; the reduction in the cost of labour through increase of tonnage is then due chiefly to the lower cost of supervision and better facilities for maintenance and repairs. Provided that the installation is of such a size as to assure reasonable economy of labour, research work and attention to the technical details of flotation are generally the most effective methods of reducing costs, since improved metallurgy is likely to result in a lower reagent consumption if not in decreased power requirements.
The costs given in Table 33 may be considered as applying to a plant built on a flat site for the two-stage selective flotation of a complex ore in subaeration machines with a tank for conditioning the pulp ahead of each stage and one cleaning operation for each rougher concentrate. It is evident that the reagent charge is by far the largest item of cost. This probably accounts for the more or less general use of machines of the mechanically agitated type for complex ores in spite of their higher power consumption and upkeep costs, since the high-speed conditioning action of the impellers and provision for the accurate regulation of each cell offer the possibility of keeping the reagent consumption at a minimum. As in the case of single-stage flotation, the charge for labour falls rapidly as the capacity of the plant increases to 1,000 tons per day ; beyond this point the rate of decrease of this and all other items of cost with increase of tonnage is less rapid. The remarks in the previous paragraph concerning the importance of research work and attention to technical details apply with added force, because of the possibility through improved metallurgy of reducing the much higher reagent and power costs which a complex ore of the class in question has to bear.