Continuing with the evaluation of copper oxide flotation using hydroxamate we now evaluate a rock that’s 41% copper oxide with some lead oxide included.
Sample B: 1.8% Copper about 41% Oxide Cu
Rougher Tests:
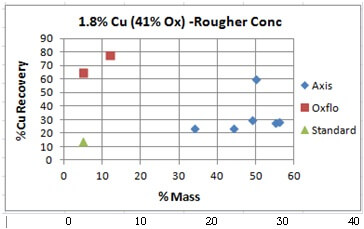
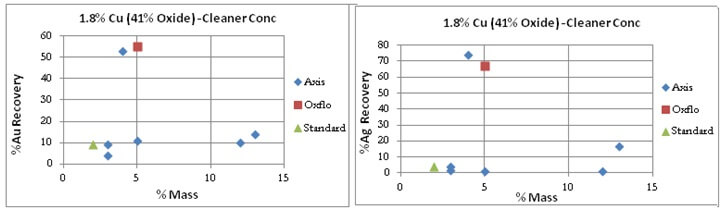
Unseen in these charts is the order in which the results were obtained. The last/best “Blue” dot (the best data point) was the last test of the series.
Oxflo’ selectivity advantage comes in with 75% Cu Recovery with only 10% Mass VS Axis’ at around 30% Recovery accompanied by an excessive 35 to 55% Mass. On its last try, Axis did manage to bring in a %Cu Recovery of %60 at a %50 Mass.
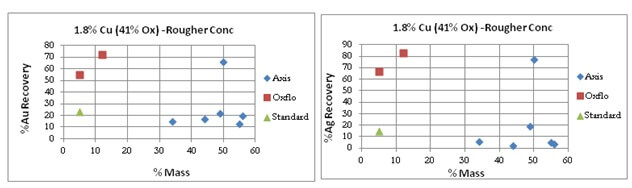
Oxflo’s selectivity for Gold/Silver is also shown by the “red” points.
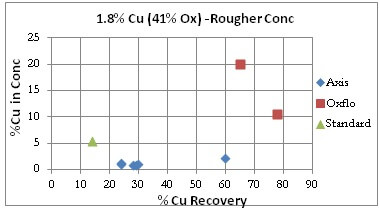
Oxflo’ superior selectivity with +%70 Cu Recoveries and the upgrade to +10% Cu conc grade produced in the Rougher circuit. Axis is unable to produce any upgrade in the rougher conc.
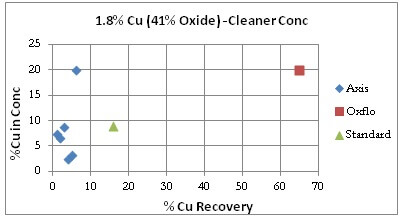
Cleaner Tests: It must be noted that Oxflo had not performed cleaner tests for the Sample Ore Type. For comparison purpose however is displayed/included one data point from Oxflo’s rougher test that had cleaner-like qualities.
The grade/recovery curve shows Axis actually under-performing the Standard with %Cu Recoveries under 10% while Oxflo sits on the far right at 65%.
After many attempts at the copper oxide flotation contest, Axis does manage to bring the results of its last test of the series to a Au/Ag %Recovery similar to Oxflo’s at 55% and 70% respectively.
Why is %Mass Recovery/Pull important
Comparing Hydroxamate Collectors
Compare Hydroxamate AM2 Rinkalore CTC3 Oxide Collectors
Oxide copper minerals generally do not respond well to traditional methods of concentration using known sulfide copper collectors. Their recovery in a froth flotation circuit requires special treatment. The traditional method involves sulfidization (at-500 to -600 mV vs. a combination Sulfide Ion Electrode) using sodium sulfide (Na2S), sodium hydrosulfide (NaSH), or ammonium sulfide ((NH4)2S) followed by flotation using xanthate or other sulfide collectors. In principle, this method is quite attractive, but in practice it suffers from two major disadvantages: a) it is difficult to control the dosage of the sulfidizing agent; an excess causes depression of both sulfide and oxide minerals, and an insufficient amount produces poor recoveries, and b) the different oxide minerals respond differently to sulfidization, and frequently sulfidization simply fails to provide acceptable oxide copper recovery.
A wide variety of collectors has been proposed for oxide copper flotation without sulfidization in the past six decades. These include a large number of organic complexing agents, fatty acids, fatty amines, and petroleum sulfonates. Except for a very limited use of fatty acids (which are quite non-selective), none of the proposed reagents has been used in an operating plant, though a large number of collectors have shown considerable promise in laboratory tests. The high cost, high consumption, and inadequate performance are three important drawbacks for the majority of the proposed collectors. Alkyl hydroxamates, however, are among the very few collectors that have shown the most promise.
In spite of the many positive attributes of hydroxamates, their large scale usage is not fully exploited. Several reasons can be proposed in order to explain this:
- The very low oxide content in the ores does not justify added cost of recovery by flotation.
- It is generally assumed that sulfidization-flotation is the preferred method.
- The use of alkyl hydroxamates has been limited to academic interest.
- Practical guidelines for the use of alkyl hydroxamates in a plant are not available. Most of the available literature has done little to provide such guidelines.
- On some ores the desired results were not obtained with hydroxamates for reasons unrelated to the chemistry of the collector.
- Oxide copper in the ore is perhaps not recoverable by flotation.
- Insufficient efforts have been made to demonstrate the efficacy and cost benefits of using alkyl hydroxamates in a plant.
It must be noted here that the discussion in this paper pertains to oxide copper that is typically associated with economical amounts of sulfide copper (the so called “mixed sulfide-oxide” ores) and, consequently, not treated by leaching-SX (the latter is widely practiced on low-grade ores from the “oxide zone” that are not treated by flotation). In specific cases where the oxide content in the mixed sulfide-oxide ore is relatively high, the tailings produced after maximum sulfide recovery, and partial oxide recovery, may be leached to recover oxide minerals that were not recovered in flotation. But for the majority of porphyry ores, this is not practiced.
The objective of this paper is to address Reasons 2-6 proposed above, and provide a clear picture of the pros- and cons- for the use of hydroxamates for oxide copper recovery.
Reason 1 is certainly beyond the scope of this paper. Furthermore, any discussion of Reason 1 is necessarily ore- or plant-specific.
Reasons 2-4 can be addressed together. In spite of the large amount of literature that exists on alkyl hydroxamates, they still appear to be rather restricted to academic research. Sulfidization-flotation has been practiced in the industry, often with some degree of success, depending on the mineralogy of the oxide minerals in the ore. Such is not the case with alkyl hydroxamates. Studies on actual ore samples from operating plants are limited. Information is lacking on the relevant practical aspects and guidelines for using alkyl hydroxamates in industrial applications. One of the objectives of this paper is to provide such practical guidelines.
Reasons 5 and 6 are related to the perceived performance of alkyl hydroxamates on “real” ores (as opposed to well-defined single minerals used in most studies) and are, therefore, of direct interest to the plant metallurgists. The extensive studies conducted by us have clearly identified conditions under which both Reasons 5 and 6 may be valid. The sometimes perceived poor performance of hydroxamates is closely related to the mineralogy of the ore, and highlights the fact that in the past investigations there was no attempt made to characterize or describe the various “oxide” minerals present in the ore under study. This aspect will be discussed fully in the paper along with supporting data from flotation and microscopy studies.
Reason 7 is also valid because there has been insufficient efforts made in the past to demonstrate the efficacy and cost benefits of using alkyl hydroxamates for a given ore type, both of which are a prerequisite for any large scale use of alkyl hydroxamates.
On the basis of several case studies considered in this paper, and flotation testing and microscopic analysis conducted on several ore samples, the following conclusions are drawn:
- Alkyl hydroxamate can effectively recover well-defined oxide copper minerals (malachite, cuprite, etc.)
- Certain AS Cu species are not amenable to flotation with hydroxamates. Examples of these are: Cu-bearing goethite, cuprite finely disseminated in gangue matrix, and chrysocollas that have a very low copper content.
- Mineralogical characterization of the ore is a prerequisite to any flotation testing. Such information will allow one to estimate the amount of copper associated with well defined oxide copper minerals and with species such as Cu-bearing goethite which are not amenable to flotation, but report as Acid Soluble Cu in chemical assays. Based on this estimate, a prediction on expected performance of hydroxamate, and an economic assessment, can be made. Chemical analysis alone is not sufficient to make such assessment because the majority of the AS Cu in the ore may not even be recoverable by flotation.
- Alkyl hydroxamates are a simpler and more attractive alternative to sulfidization-flotation.
- Microscopically, to the untrained eye, Cu-bearing goethite can be misidentified as cuprite, and this could lead one to the false conclusion that hydroxamates could not float cuprite.
- Assay methods for AS Cu are not standardized. Many different methods are used. The assay numbers, therefore, have ambiguity associated with them. Chemical assays must, therefore, be complemented with mineralogical analysis.